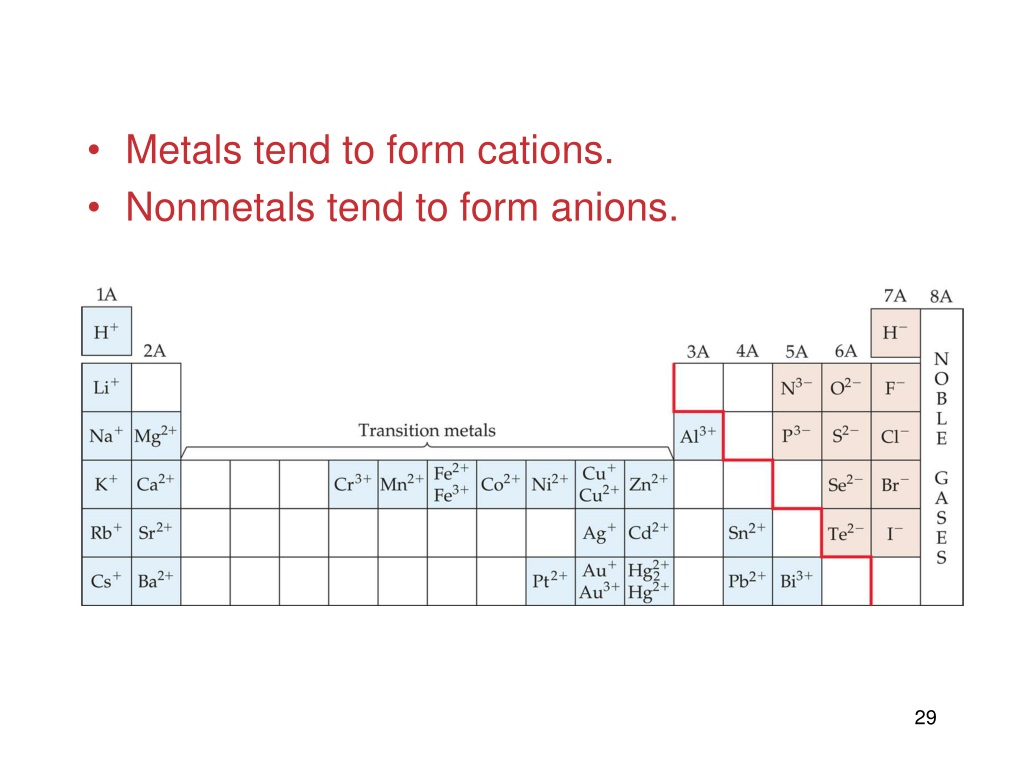
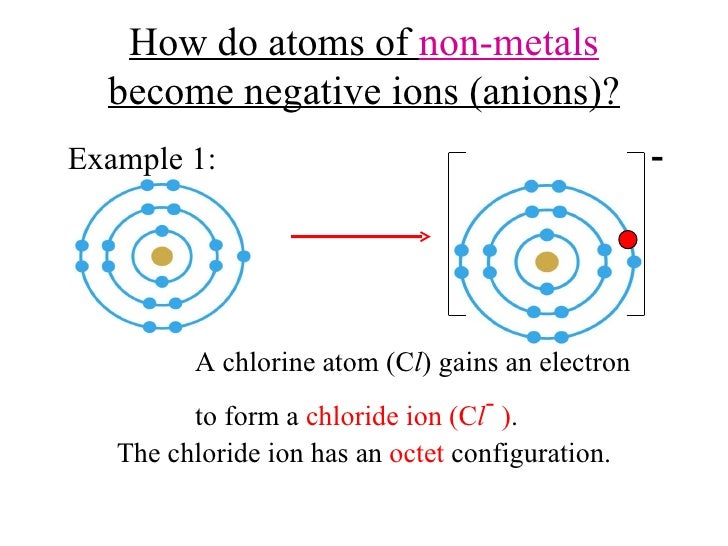
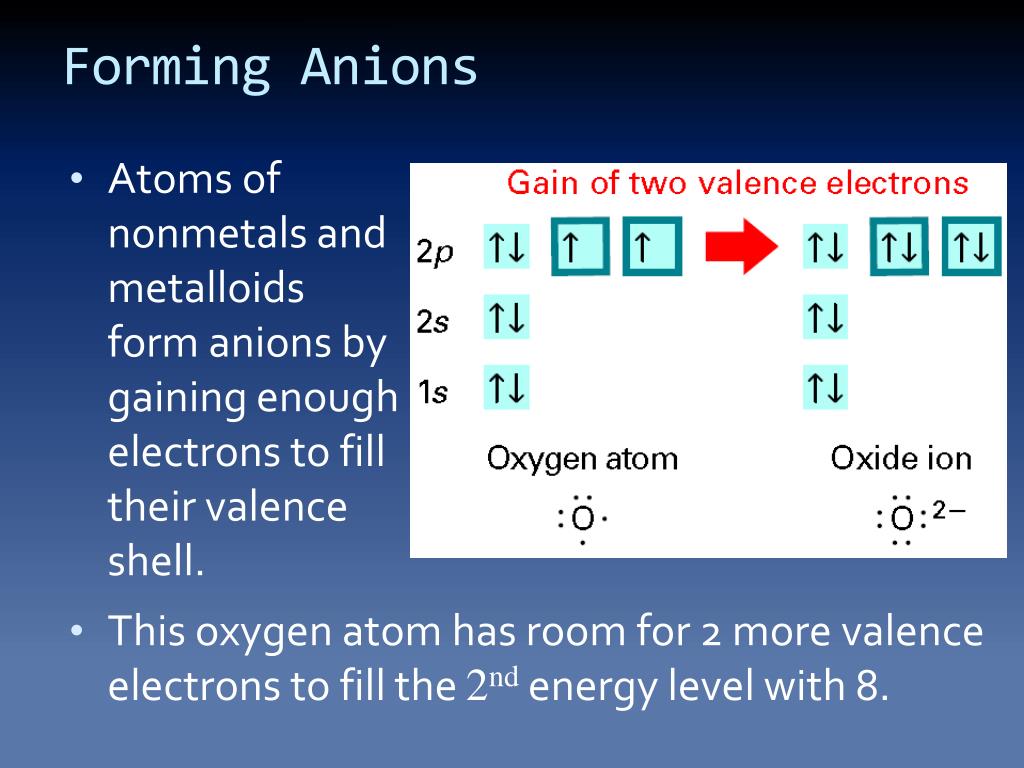
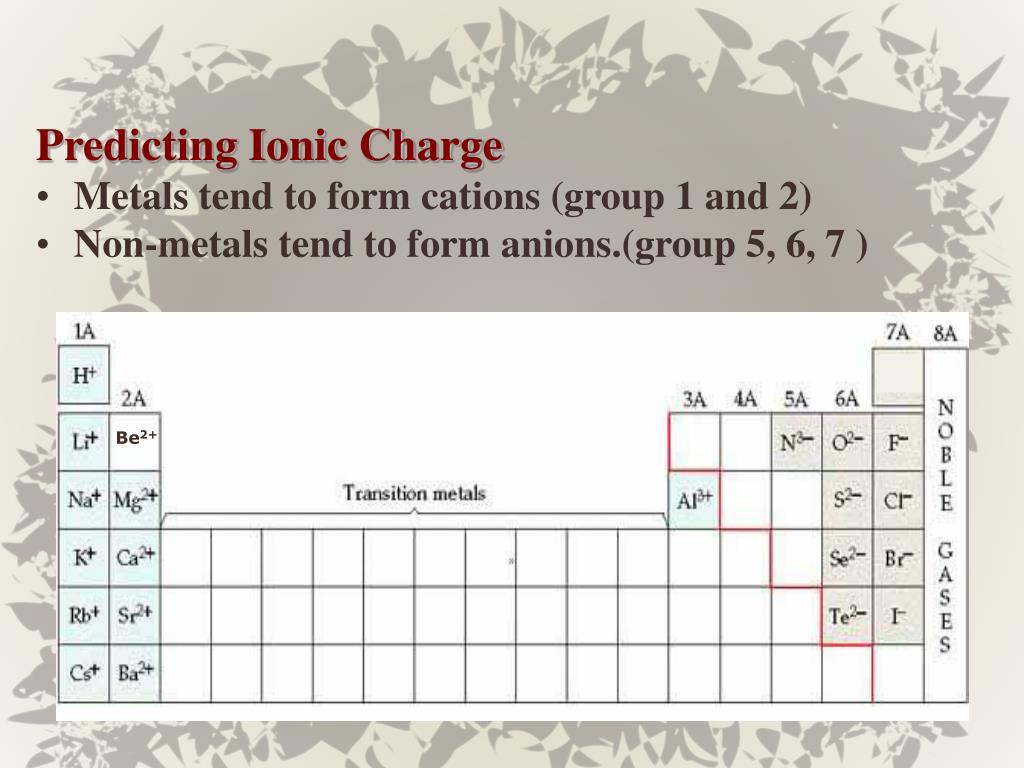
Why Do Nonmetals Tend To Form Anions
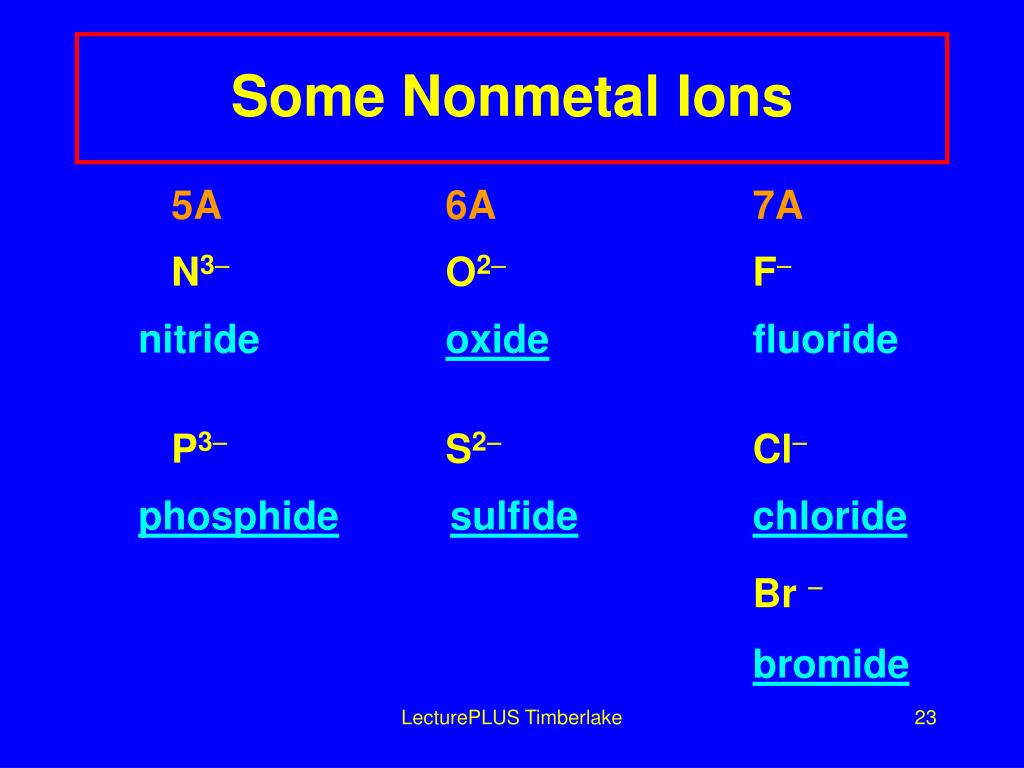
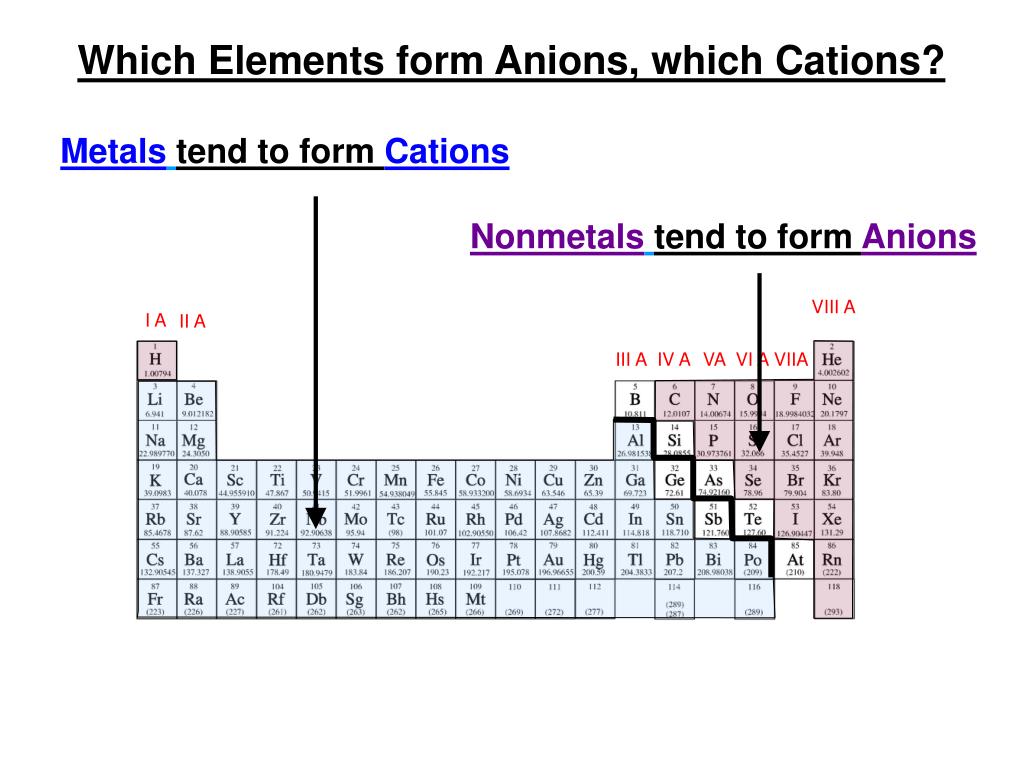
Why Do Nonmetals Tend To Form Anions - Metals tend to form cations, while nonmetals tend to form anions. Web most nonmetals become anions when they make ionic compounds. An electron is gained state the number of electrons lost or. Solution verified create an account to view solutions by signing up, you accept quizlet's. A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is. Nonmetals like to gain electrons to form anions to have a fully stable octet. Web created by hermy456 terms in this set (30) two ways an ion forms from an atom. They generally have more than 4 valence electrons at the time, so it would be easier to gain. Web this is actually one of the chemical properties of metals and nonmetals: It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web created by hermy456 terms in this set (30) two ways an ion forms from an atom. Web we would like to show you a description here but the site won’t allow us. Web question explain why metals tend to form cations, while. Energy is released when electrons are removed from metal ions. Second, elements that live in the first two. Metals tend to form cations, while nonmetals tend to form anions. They release energy (exothermic) to gain electrons to form an anion; Nonmetals like to gain electrons to form anions to have a fully stable octet. They generally have more than 4 valence electrons at the time, so it would be easier to gain. Web this is actually one of the chemical properties of metals and nonmetals: An electron is gained state the number of electrons lost or. These are electronegative elements with high ionization energies. A neutral chlorine atom has seven electrons in its outermost. Energy is released when electrons are removed from metal ions. Web we would like to show you a description here but the site won’t allow us. Solution verified create an account to view solutions by signing up, you accept quizlet's. Web why do nonmetals tend to form anions rather than cations? Web (i) atomic radii decrease across the period from. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Only one more electron is. Web why do nonmetals tend to form anions when they react to form compounds? Web thus, nonmetals tend to form negative ions. Web this is actually one of the chemical properties of metals and nonmetals: Web we would like to show you a description here but the site won’t allow us. These are electronegative elements with high ionization energies. Web created by hermy456 terms in this set (30) two ways an ion forms from an atom. Web this is actually one of the chemical properties of metals and nonmetals: Second, elements that live in the. Web question explain why metals tend to form cations, while nonmetals tend to form anions. Web this is actually one of the chemical properties of metals and nonmetals: Web created by hermy456 terms in this set (30) two ways an ion forms from an atom. Energy is released when electrons are removed from metal ions. It is easiest to achieve. A neutral chlorine atom has seven electrons in its outermost shell. Web created by hermy456 terms in this set (30) two ways an ion forms from an atom. Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Solution. Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Energy is released when electrons are removed from metal ions. Web we would like to show you a description here but the site won’t allow us. They generally have. Metals tend to form cations, while nonmetals tend to form anions. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. These are electronegative elements with high ionization energies. Web question why do nonmetals tend to form anions rather than cations? Nonmetals like to gain electrons to form. A neutral chlorine atom has seven electrons in its outermost shell. Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. 1 metals have low lonization. Web this is actually one of the chemical properties of metals and nonmetals: Solution create an account to view solutions recommended textbook solutions introduction to chemical. Web question why do nonmetals tend to form anions rather than cations? Only one more electron is. Energy is released when electrons are removed from metal ions. Metals tend to form cations, while nonmetals tend to form anions. Web we would like to show you a description here but the site won’t allow us. An electron is gained state the number of electrons lost or. Web we would like to show you a description here but the site won’t allow us. Web question explain why metals tend to form cations, while nonmetals tend to form anions. Web why do nonmetals tend to form anions rather than cations? Web why do nonmetals tend to form anions when they react to form compounds? Web thus, nonmetals tend to form negative ions. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web the nonmetals have higher electronegativities than do metals, and compounds formed between metals and nonmetals are generally ionic in nature because of the large. Web created by hermy456 terms in this set (30) two ways an ion forms from an atom. Second, elements that live in the first two.PPT Chapter 7 Periodic Properties of the Elements PowerPoint
Chem matters ch6_ionic_bond
PPT IONS PowerPoint Presentation, free download ID2435906
PPT IONS PowerPoint Presentation, free download ID2435906
PPT Atoms, Molecules and Ions PowerPoint Presentation, free download
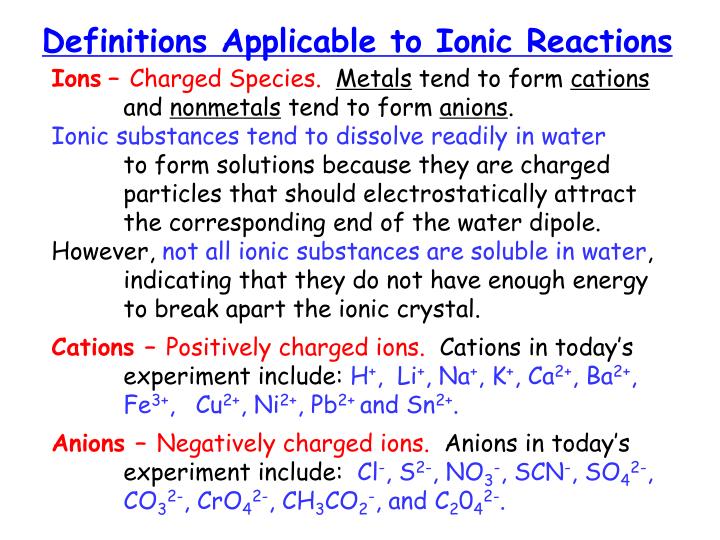
PPT Mystery of the 15 Test Tubes PowerPoint Presentation ID2995481
Cations and Anions Definitions, Examples, and Differences
PPT Chemical Bonds PowerPoint Presentation, free download ID930911
PPT Lecture 4. Chapter 2. Structure of the Atom (Contd.) PowerPoint
PPT Ionic Bonds PowerPoint Presentation, free download ID1990542
Related Post: