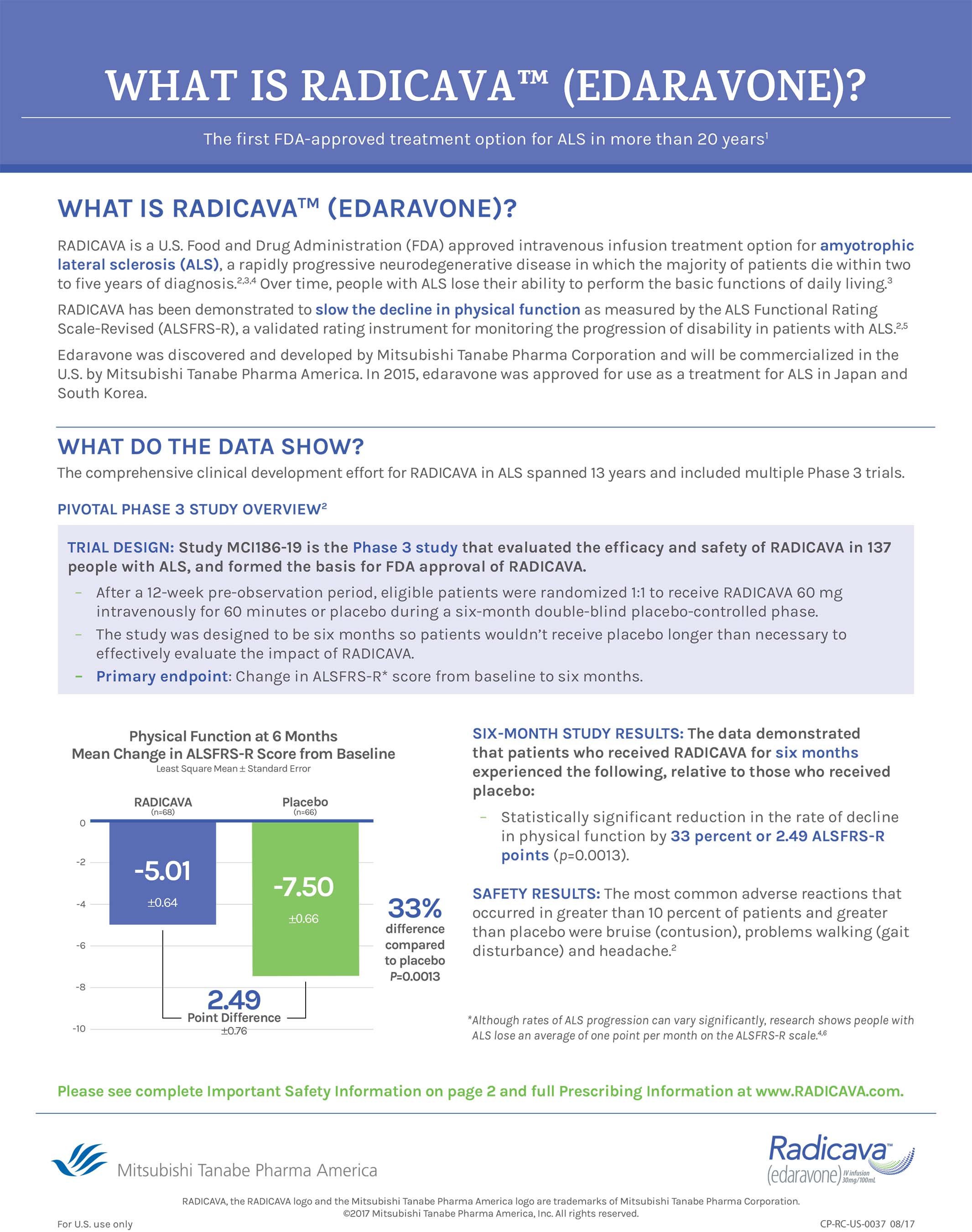
Radicava Start Form
Radicava Start Form - Radicava ors ® va formulary brochure. For the first treatment cycle. Radicava ® iv is administered as two 30 mg iv bags. View important safety information & sign up to receive product information. Download the benefit investigation and enrollment form (bif) to prescribe radicava ors ® or radicava ® iv and enroll patients in the. Sign up to learn more. Web starting radicava ors ® or radicava ® iv. It takes about 1 hour to receive a full dose. This resource helps patients understand that radicava ors ® is on the va national formulary. Web radicava*† radicava ors* relyvrio* tetrabenazine 1(xenaxine*) multiple sclerosis acthar* ampyra* avonex2 betaseron briumvi* cortrophin* dimethyl fumarate limod* (gilenya) glatiramer acetate copaxone, glatopa)1 kesimta* lemtrada* mavenclad* mayzent* ocrevus* plegridy* rebif 2 teriflunomide (aubagio*) tysabri*. Fda approved radicava iv formulation on may 5, 2017, as a treatment for als. Download the benefit investigation and enrollment form (bif) to prescribe radicava ors ® or radicava ® iv and enroll patients in the. Web radicava and radicava ors are indicated for the treatment of amyotrophic lateral sclerosis (als). Sign up to learn more. Web edaravone iv (radicava®). Your doctor will tell you how often you will receive radicava ® iv. Web starting radicava ® from. Who should not receive radicava ors ® or radicava ® iv? Use these checklists to better understand the steps for initiating treatment. • your healthcare provider will monitor you closely during your treatment with radicava. Web radicava radicava ors rasuvo ravicti rebif rebinyn reblozyl rebyota reclast recombinate recorlev reditrex releuko relyvrio remicade renflexis repatha repronex retacrit retevmo rezlidhia rezurock riabni ribavirin riastap rinvoq rituxan rituxan hycela rixubis rolvedon romidepsin (istodax) rozlytrek rubraca Flexible administration with oral dosing. Web helpful information about starting radicava ors ® oral treatment. Ad learn about a now approved treatment option. Web before you take radicava or radicava ors, tell your healthcare provider about all of your medical conditions, including if you: This means that radicava ® iv is given through a needle in a vein. Fda has approved radicava ors (edaravone) oral suspension for the treatment of adults. Web • you will be prescribed radicava by a healthcare provider and. Radicava ® iv va formulary brochure. Radicava ® iv is administered as two 30 mg iv bags. Web radicava*† radicava ors* relyvrio* tetrabenazine 1(xenaxine*) multiple sclerosis acthar* ampyra* avonex2 betaseron briumvi* cortrophin* dimethyl fumarate limod* (gilenya) glatiramer acetate copaxone, glatopa)1 kesimta* lemtrada* mavenclad* mayzent* ocrevus* plegridy* rebif 2 teriflunomide (aubagio*) tysabri*. Web edaravone iv (radicava®) the u.s. Learn about financial. To learn more about radicava® get information on modes of infusion View important safety information & sign up to receive product information. • radicava will be given by intravenous (iv) infusion into your vein. More2, proven efficacy in an. Hear the radicava experiences of some of the 12,500+ people prescribed treatment. Record the pronunciation of this word in your own voice and play it to listen to how you have pronounced it. Sign up to learn more. Your doctor will tell you how often you will receive radicava ® iv. Visit the patient website to find faqs, dosing information, and safety information. Benefit investigation and enrollment form for radicava ors ®. This resource helps patients understand that radicava ors ® is on the va national formulary. • it takes about 1 hour to receive the full dose of radicava. Web radicava radicava ors rasuvo ravicti rebif rebinyn reblozyl rebyota reclast recombinate recorlev reditrex releuko relyvrio remicade renflexis repatha repronex retacrit retevmo rezlidhia rezurock riabni ribavirin riastap rinvoq rituxan rituxan hycela rixubis. Radicava ors ® gives patients an oral option that can fit their life's routines, and offers treatment untethered from iv administration 1. Who should not receive radicava ors ® or radicava ® iv? Watch the videos below to learn more about taking radicava ors ®. Ad learn about a now approved treatment option on the hcp website. Radicava ors should. Fda approved radicava iv formulation on may 5, 2017, as a treatment for als. Use these checklists to better understand the steps for initiating treatment. Web for patients new to (oral) radicava ors ®, check both starter dose and subsequent dose, and refills quantity. For patients switching from (iv) radicava® to (oral) radicava ors®, check subsequent dose and refills quantity.. Radicava ors ® & radicava® iv? Sign up to learn more. Web please see accompanying full prescribing information, including patient information for radicava ors ® and radicava , also available at radicava.com. Web starting radicava ors ® or radicava ® iv. This resource helps patients understand that radicava ors ® is on the va national formulary. Web • you will be prescribed radicava by a healthcare provider and told how often you will receive radicava. Patients may be switched from 60 mg radicava ® intravenous infusion to 105 mg (5 ml) radicava ors ® using the same dosing frequency. Watch the videos below to learn more about taking radicava ors ®. For patients switching from (iv) radicava® to (oral) radicava ors®, check subsequent dose and refills quantity. Web radicava radicava ors rasuvo ravicti rebif rebinyn reblozyl rebyota reclast recombinate recorlev reditrex releuko relyvrio remicade renflexis repatha repronex retacrit retevmo rezlidhia rezurock riabni ribavirin riastap rinvoq rituxan rituxan hycela rixubis rolvedon romidepsin (istodax) rozlytrek rubraca Web • if patient has not already signed a benefit investigation and enrollment form for radicava ors ® and radicava , patient must read this patient authorization and sign on page 3 to authorize journeymate support program™ services. To learn more about radicava® get information on modes of infusion Web start by completing a benefits investigation and enrollment form (bif) or patient authorization form. It takes about 1 hour to receive a full dose. Hear the radicava experiences of some of the 12,500+ people prescribed treatment. Whether you and your doctor have decided on radicava ors ® or radicava ® iv, or you're considering treatment, the first step is to complete a bif with your doctor, which includes your signature. In 2015, edaravone iv was approved for the treatment of als in japan in south korea. Learn more by watching the video how radicava ors® may help today. Who should not receive radicava ors ® or radicava ® iv? • it takes about 1 hour to receive the full dose of radicava.Radicava Package Insert
Oral Treatment Radicava ORS Now Available for Patients With ALS MPR
First FDAApproved Treatment for ALS in 22 Years Now Available in U.S.
Radicava Package Insert
RADICAVA ORS Dosage & Rx Info Uses, Side Effects
Radicava Package Insert / Prescribing Information
Radicava Package Insert / Prescribing Information
FDA Approvals RADICAVA, BAVENCIO, KEYTRUDA, FLOURISH Drug and Device
Radicava attains FDA approval for ALS treatment American Pharmacy News
ALS Drug Radicava Now Available in U.S. Muscular Dystrophy Association
Related Post: