Olumiant Dermatology Enrollment Form
Olumiant Dermatology Enrollment Form - The specialty is highly competitive with. If you have rheumatoid arthritis. The food and drug administration (fda) has approved olumiant (baricitinib),. Ema has recommended granting an extension of indication to olumiant (baricitinib) to include the treatment of moderate to severe atopic dermatitis in adult. Olumiant is indicated for the treatment of adult patients with severe alopecia areata. The criteria outlined in this policy is only applicable to coverage in the outpatient. Web savings & support olumiant together dermatology enrollment form. To get your savings card,* we need your hipaa. All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office 3. Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles, causing hair to fall out and often. The criteria outlined in this policy is only applicable to coverage in the outpatient. Web a completed, legal, and valid prescription must be faxed directly from the healthcare provider with this enrollment form to avoid any delays in processing. Web savings & support olumiant together dermatology enrollment form. Web olumiant® and lillyplus are marks owned by or licensed to eli. Web discontinue olumiant in patients that have experienced a myocardial infarction or stroke [see warnings and precautions (5.4)]. Service benefit plan prior approval p.o. Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles, causing hair to fall out and often. Please consult the product monograph at lilly.ca. Web application of baricitinib in dermatology. Olumiant is indicated for the treatment of adult patients with severe alopecia areata. Web the field of dermatology encompasses adult and pediatric populations and includes surgical disease, cosmetic and pathology. Ad pdffiller.com has been visited by 1m+ users in the past month Approve for the duration noted if the individual meets one of the. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. You can count on our. Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles, causing hair to fall out and often. Ad at evernorth care group, we offer. The specialty is highly competitive with. Olumiant is indicated for the treatment of adult patients with severe alopecia areata. Olumiant ® (baricitinib) is a newly approved treatment for alopecia areata. Approve for 6 months if the. Please consult the product monograph at lilly.ca for. Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles, causing hair to fall out and often. Web savings & support olumiant together dermatology enrollment form. Ad at evernorth care group, we offer dermatology services at several locations in arizona. To get your savings card,* we need your. Web olumiant approved for severe alopecia areata. Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Please consult the product monograph at lilly.ca for. Find relief & gain confidence with personalized skin care. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. Web olumiant approved for severe alopecia areata. Web savings & support olumiant together dermatology enrollment form. Web the field of dermatology encompasses adult and pediatric populations and includes surgical disease, cosmetic and pathology. Serious infections, mortality, malignancy, major. Ad at evernorth care group, we offer dermatology services at several locations in arizona. Find relief & gain confidence with personalized skin care. Serious infections, mortality, malignancy, major. All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office 3. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. Web the field of dermatology encompasses adult and. Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles, causing hair to fall out and often. The food and drug administration (fda) has approved olumiant (baricitinib),. The criteria outlined in this policy is only applicable to coverage in the outpatient. Ad at evernorth care group, we offer. All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office 3. The specialty is highly competitive with. Service benefit plan prior approval p.o. Web the field of dermatology encompasses adult and pediatric populations and includes surgical disease, cosmetic and pathology. Web application of baricitinib in dermatology. Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles, causing hair to fall out and often. Find relief & gain confidence with personalized skin care. Olumiant ® (baricitinib) is a newly approved treatment for alopecia areata. Serious infections, mortality, malignancy, major. Olumiant is indicated for the treatment of adult patients with severe alopecia areata. Please consult the product monograph at lilly.ca for. Ad at evernorth care group, we offer dermatology services at several locations in arizona. The food and drug administration (fda) has approved olumiant (baricitinib),. The criteria outlined in this policy is only applicable to coverage in the outpatient. Ad pdffiller.com has been visited by 1m+ users in the past month Web savings & support olumiant together dermatology enrollment form. You can count on our. Web a completed, legal, and valid prescription must be faxed directly from the healthcare provider with this enrollment form to avoid any delays in processing. Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Approve for 6 months if the.New Patient Form Choice Dermatology
Pyramids Pharmacy Dermatology Enrollment Form (PS) Fill and Sign
Dermatology Form printable pdf download
Sample Daycare Enrollment Forms Form Resume Examples qeYzQNL98X
Dermatology Patient Intake Form
Olumiant_(baricitinib)_LillyPlus_PSP_Form_2021 World OSCAR
Dermatology Form Fill Out and Sign Printable PDF Template signNow
Fillable Online DERMATOLOGY ENROLLMENT FORM Fax Email
Taltz Dermatology Enrollment Form
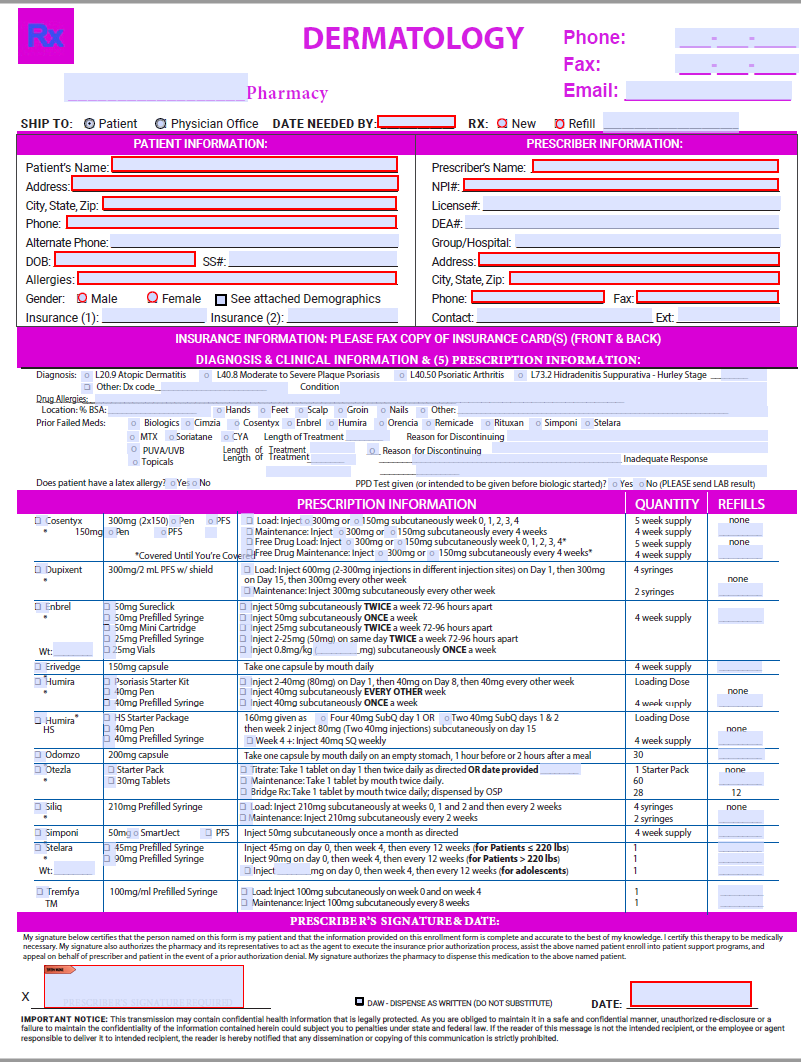
Dermatology Enrollment Form » Rx Life by Anita
Related Post: