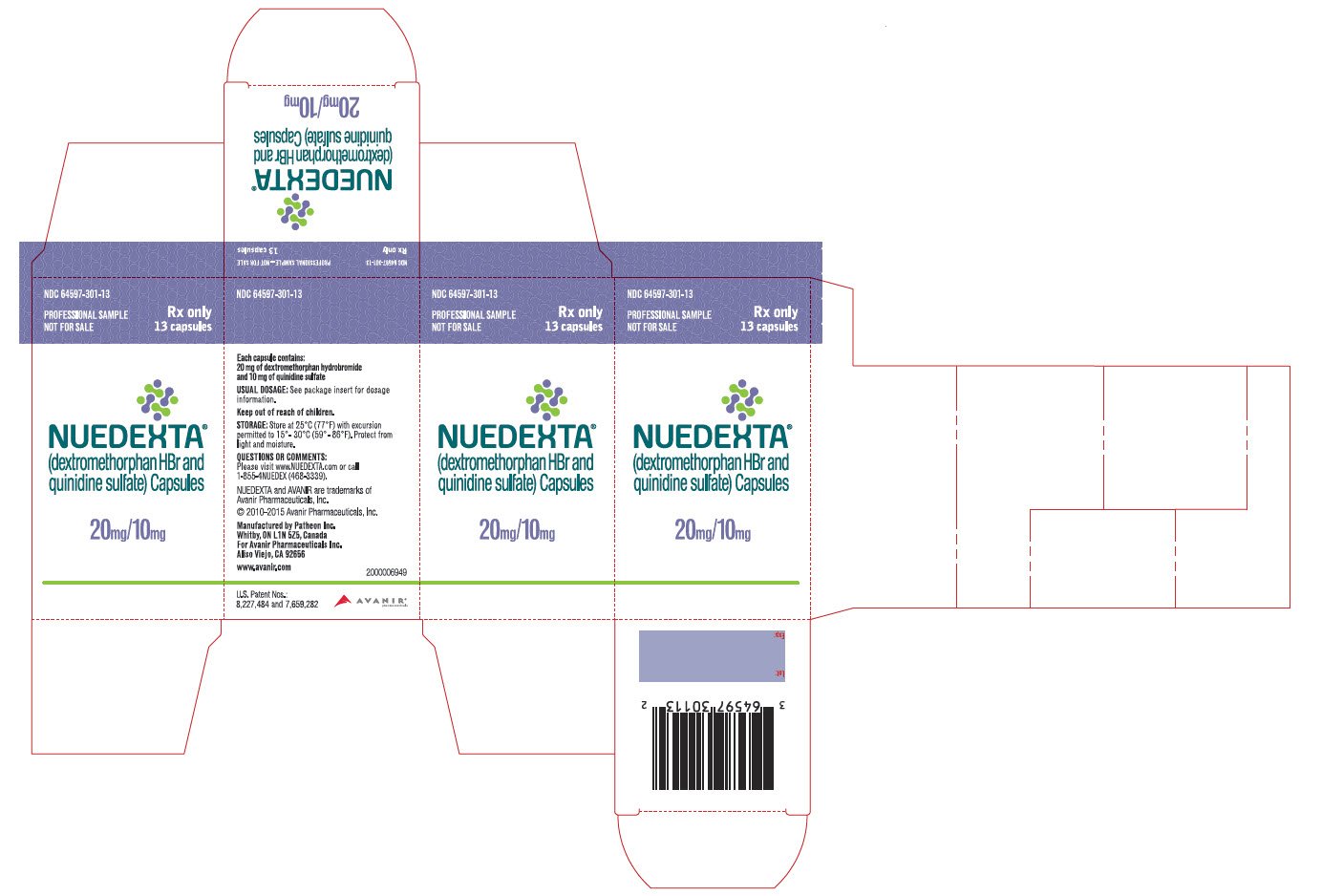
Nuedexta Enrollment Form
Nuedexta Enrollment Form - Are you a patient or a caregiver? Web up to $3 cash back forms: 2 following this regimen, patients on nuedexta. Web confirmation that you have successfully signed up for information about nuedexta® (dextromethorphan hbr and quinidine sulfate) 20 mg/10 mg capsules. Web to expedite the application process, healthcare professionals and patients may fill out and submit an application with all requested documentation online via the opaf care. See your medicare advantage annual open enrollment options. Efficacy beyond week 1 in the star pivotal trial was achieved with q12h dosing. Learn what nuedexta is indicated to treat and important safety information to follow. Web our pseudobulbar affect (pba) and nuedexta resource library can support you and your care teams in identifying pba at your practice or facility, talking to your patients and their. Enrollment application for samsca ® (tolvaptan) tablets. Please fill out the form below. Web our pseudobulbar affect (pba) and nuedexta resource library can support you and your care teams in identifying pba at your practice or facility, talking to your patients and their. Web please refer to your supplemental new drug application (snda) dated july 31, 2014, received august 1, 2014, submitted pursuant to section 505(b)(2) of. Learn what nuedexta is indicated to treat and important safety information to follow. Web how it works. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Shipment will contain 8 nuedexta samples/bottles. One month prior to their enrollment ending, a. Yes (first approved october 29, 2010) brand name: Web how it works. All fields below are required. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Web complete the form in its entirety including: Learn what nuedexta is indicated to treat and important safety information to follow. Ad singlecare.com has been visited by 100k+ users in the past month Web please refer to your supplemental new drug application (snda) dated july 31, 2014, received august 1, 2014, submitted pursuant to section 505(b)(2) of the federal food,. State license number (no abbreviations,. All fields below. Enroll in a private medicare advantage plan today! Ad singlecare.com has been visited by 100k+ users in the past month Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. See your medicare advantage annual open enrollment options. Web please refer to your supplemental new drug application (snda) dated july 31, 2014, received august 1, 2014, submitted pursuant to section 505(b)(2) of the federal food,. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Web nuedexta fda approval history. 2 following this regimen, patients on nuedexta. Please fill out the form below. State license number (no abbreviations,. Efficacy beyond week 1 in the star pivotal trial was achieved with q12h dosing. Please fill out the form below. 2 following this regimen, patients on nuedexta. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Web complete the form in its entirety including: Web nuedexta fda approval history. Enroll in a private medicare advantage plan today! 2 following this regimen, patients on nuedexta. Web up to $3 cash back forms: Web confirmation that you have successfully signed up for information about nuedexta® (dextromethorphan hbr and quinidine sulfate) 20 mg/10 mg capsules. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Ad singlecare.com has been visited by 100k+ users in the past month Web nuedexta fda approval history. State license number (no abbreviations,. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Learn what nuedexta is indicated to treat and important safety information to follow. Web enrollment application for nuedexta ® (dextromethorphan hbr and quinidine sulfate) capsules. Efficacy beyond week 1 in the star pivotal trial was achieved with q12h dosing. Shipment will contain 8 nuedexta samples/bottles. Indication and important safety information for. 2 following this regimen, patients on nuedexta. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Learn what nuedexta is indicated to treat and important safety information to follow. What are common nuedexta doses? Web nuedexta fda approval history. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Web up to $3 cash back forms: What form (s) does nuedexta come in? Web how it works. Ad nuedexta® (dextromethorphan hbr and quinidine sulfate) 20mg/10mg capsules. Ad singlecare.com has been visited by 100k+ users in the past month Are you a patient or a caregiver? See your medicare advantage annual open enrollment options. Are you a patient or a caregiver? All fields below are required. Web our pseudobulbar affect (pba) and nuedexta resource library can support you and your care teams in identifying pba at your practice or facility, talking to your patients and their. Web complete the form in its entirety including: Patient must attach a copy of. Web nuedexta ® sample request form.Registered Child Care Home Texas aregoingwell
Nuedexta FDA prescribing information, side effects and uses
Fillable 2010 High School Enrollment Form printable pdf download
Fillable Student Enrollment Form printable pdf download
Kaiser permanente enrollment forms
Daycare Enrollment Forms Free Form Resume Examples gq96gRxYOR
PFT Member Enrollment Form Philadelphia Federation of Teachers
DepEd Basic Education Enrollment Form TeacherPH
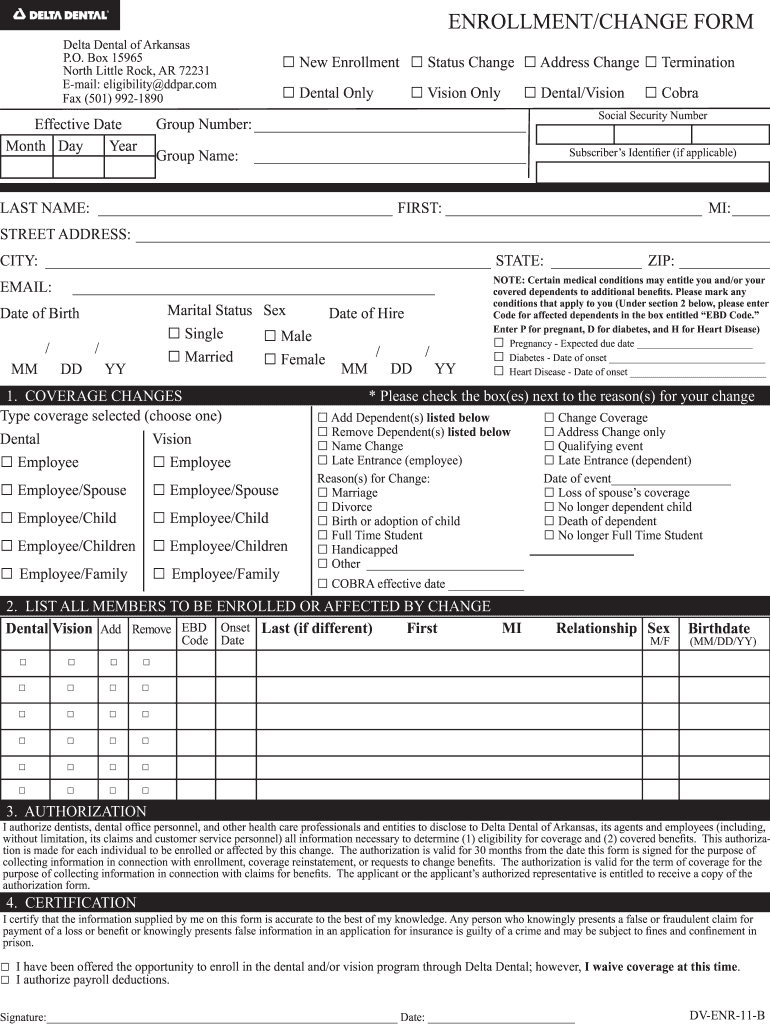
Delta Dental Enrollment Change Form Fill Online, Printable, Fillable
Study Raises Questions on Nuedexta Prescribing MedPage Today
Related Post: