Nitrogen Gas And Hydrogen Gas React To Form Ammonia
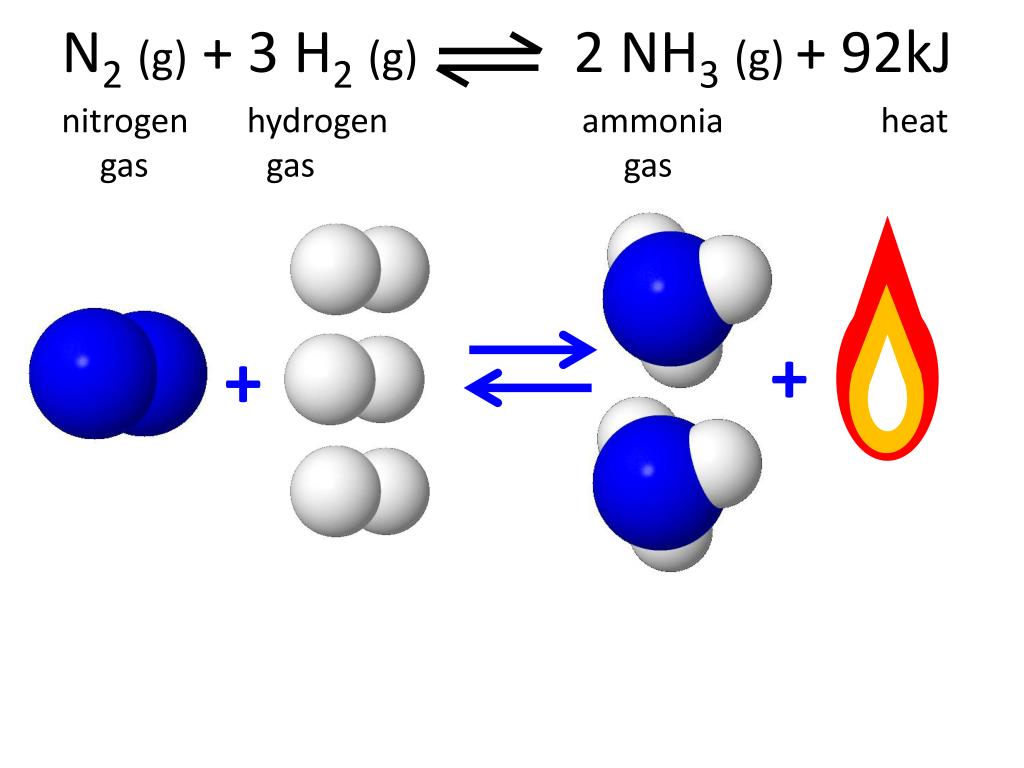
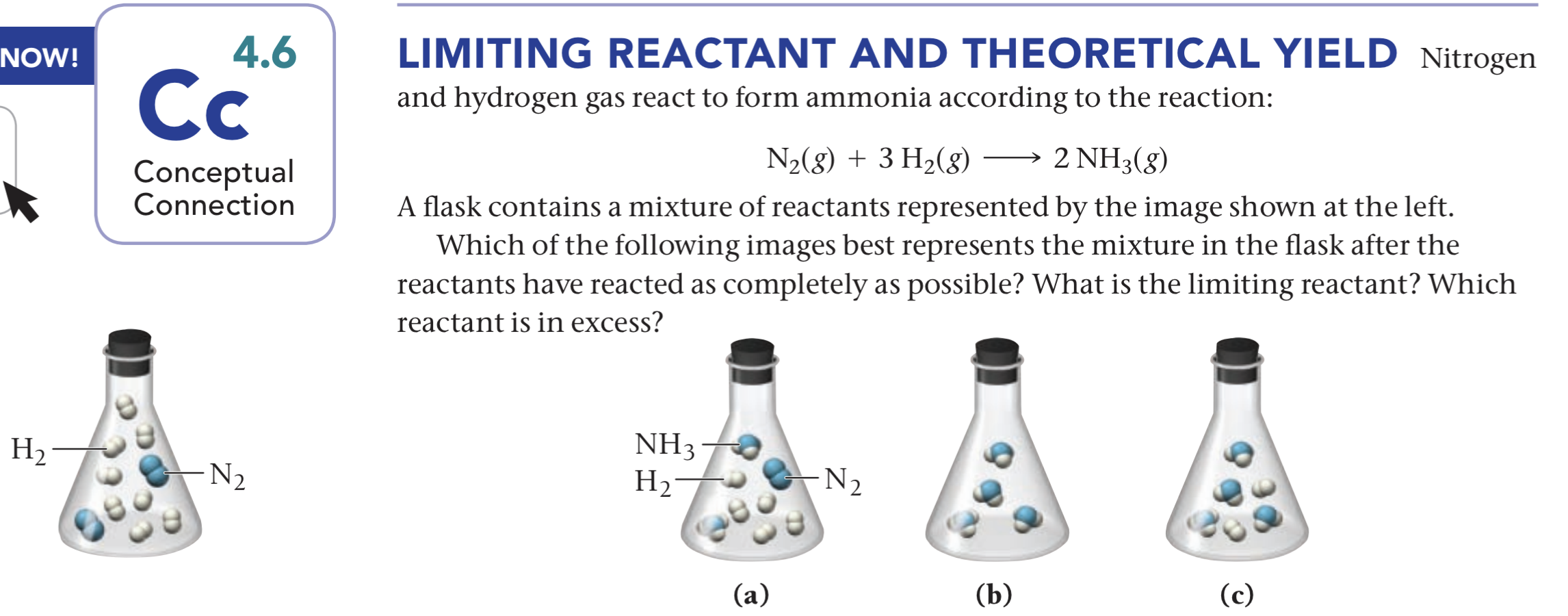
Nitrogen Gas And Hydrogen Gas React To Form Ammonia - Nitrogengas and hydrogengas react to form ammoniagas. What volume of carbon etrachloride would be produced by this. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Identify all of the phases in your answer. Web if a reaction just involves gases, then the coefficients can be used to show relationships between moles of reactant and products and also leaders of reactant and products,. Thus, 7.7 ml = 0.0077 l. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: The volume of nitrogen (n₂) gas = 7.7 ml. 2 moles of hydrogen gas are mixed with 4 moles of. Suppose you have 11.0 molof n2 and 9.0 mol of h2 in a reactor. Suppose you have 11.0 molof n2 and 9.0 mol of h2 in a reactor. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3. N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the. 2 moles n2 * (3 moles h2. What volume of ammonia would be produced by this reaction if 5,4 ml. 2 moles of hydrogen gas are mixed with 4 moles of. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: What volume of carbon etrachloride would be produced by this. Web nitrogen monoxide gas reacts with hydrogen gas to form ammonia gas. Express your answer as a chemical equation. 1 ml = 0.001 l. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web nitrogen monoxide gas reacts with hydrogen gas to form ammonia gas and water vapor. The volume of nitrogen (n₂) gas = 7.7 ml. Hydrogen gas and nitrogen gas react to form ammonia gas. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. What volume of ammonia would be produced by this reaction if 6.9 m3 of. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Suppose. N2( g)+3h2( g) → 2nh3( g) if all the n2 and h2 are consumed, what volume of nh3, at the. Web science methane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. Web ammonia is created in the haber process in a rigid container (nitrogen gas plus hydrogen gas react to form ammonia gas). The. Hydrogen gas and nitrogen gas react to form ammonia gas. Nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2 reacts with 3.3 l. Web ammonia is created in the haber process in a rigid container (nitrogen gas plus hydrogen. Identify all of the phases in your answer. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2 reacts with 3.3 l. Could half the n2 react?. N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the. The volume of nitrogen (n₂) gas = 7.7 ml. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: What volume of ammonia would be produced by this reaction if 6.9 m3 of. Hydrogen gas and nitrogen gas react to form ammonia gas. Could half the n2 react?. N2 (g) + 3h2 (g) +2nh3 (g) at a certain. Web if a reaction just involves gases, then the coefficients can be used to show relationships between moles of reactant and products and also leaders of reactant and products,. Hydrogen gas and nitrogen gas react to form ammonia gas. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Hydrogen gas and nitrogen gas react to. What volume of carbon etrachloride would be produced by this. Hydrogen gas and nitrogen gas react to form ammonia gas. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; What volume of ammonia would be produced by this reaction if 5,4 ml. Web ammonia is created in the. Hydrogen gas and nitrogen gas react to form ammonia gas. Suppose you have 11.0 molof n2 and 9.0 mol of h2 in a reactor. Express your answer as a chemical equation. N2( g)+3h2( g) → 2nh3( g) if all the n2 and h2 are consumed, what volume of nh3, at the. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2 reacts with 3.3 l. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3. The volume of nitrogen (n₂) gas = 7.7 ml. Web if a reaction just involves gases, then the coefficients can be used to show relationships between moles of reactant and products and also leaders of reactant and products,. Identify all of the phases in your answer. What volume of carbon etrachloride would be produced by this. Hydrogen gas and nitrogen gas react to form ammonia gas. Suppose you have 13.0 mol n2 of and 9.0 h2 of in a reactor. Web ammonia is created in the haber process in a rigid container (nitrogen gas plus hydrogen gas react to form ammonia gas). Web chemistry questions and answers. Thus, 7.7 ml = 0.0077 l. What volume of ammonia would be produced by this reaction if 6.9 m3 of. What volume of ammonia would be produced by this reaction if 5,4 ml. Nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: 2 moles of hydrogen gas are mixed with 4 moles of.Solved Nitrogen (N2) and hydrogen (H2) react to form ammonia
[Solved] Hydrogen gas and nitrogen gas can react to form ammonia
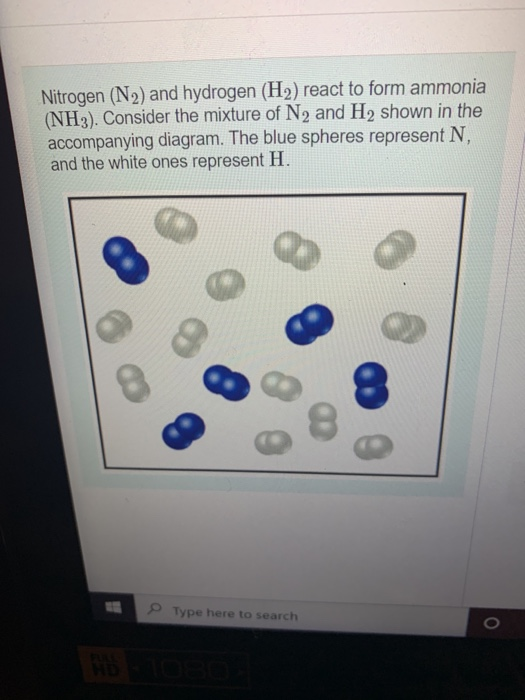
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
PPT Reaction Rates PowerPoint Presentation, free download ID6516140
Beautiful Work Nitrogen Reacts With Hydrogen Equation Of Respiration
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
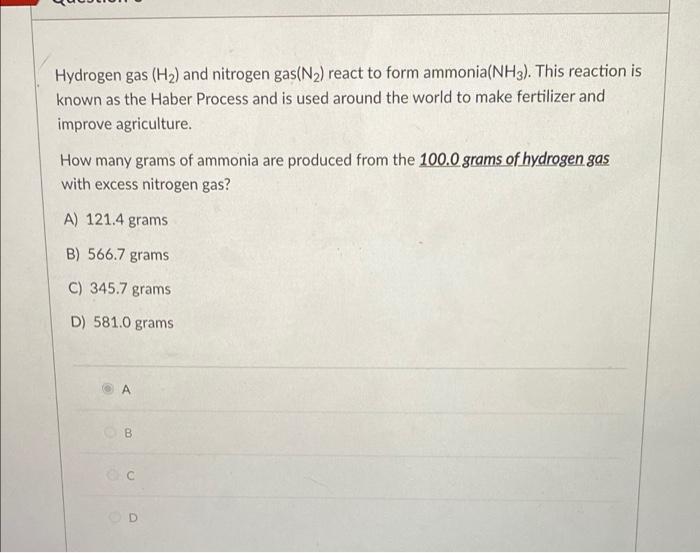
Solved Hydrogen gas (H2) and nitrogen gas(N2) react to form
⚗️Question 6 (1 point) Hydrogen gas and nitrogen gas can react to form
Nitrogen Gas Reacts With Hydrogen Gas To Form Ammonia Printable Form
⚗️Hydrogen gas and nitrogen gas can react to form ammonia according to
Related Post: