Ne Electron Configuration Long Form
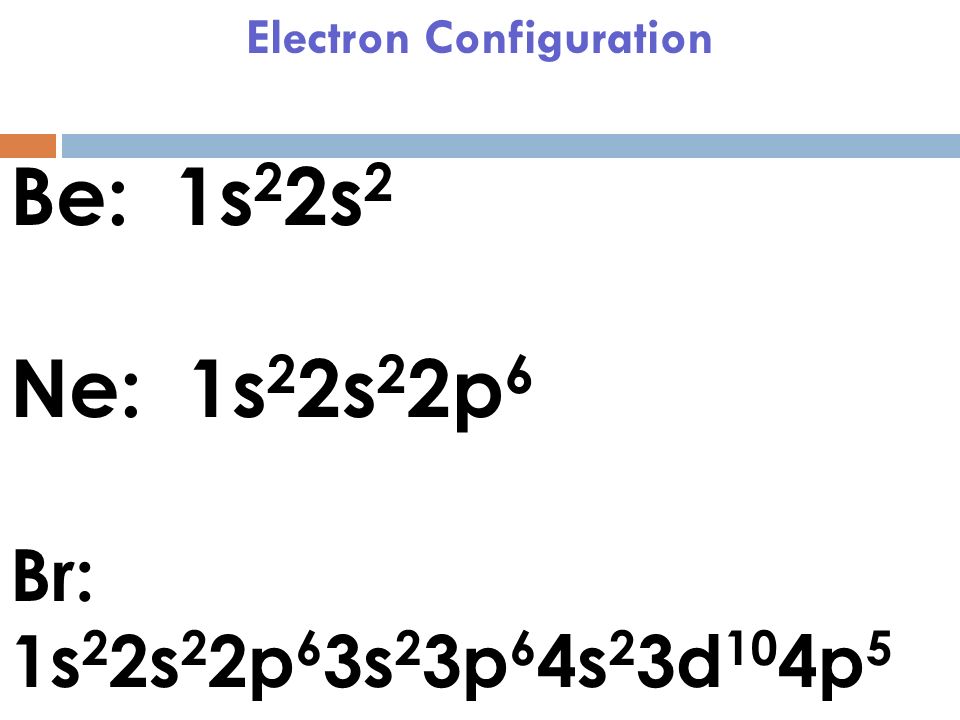
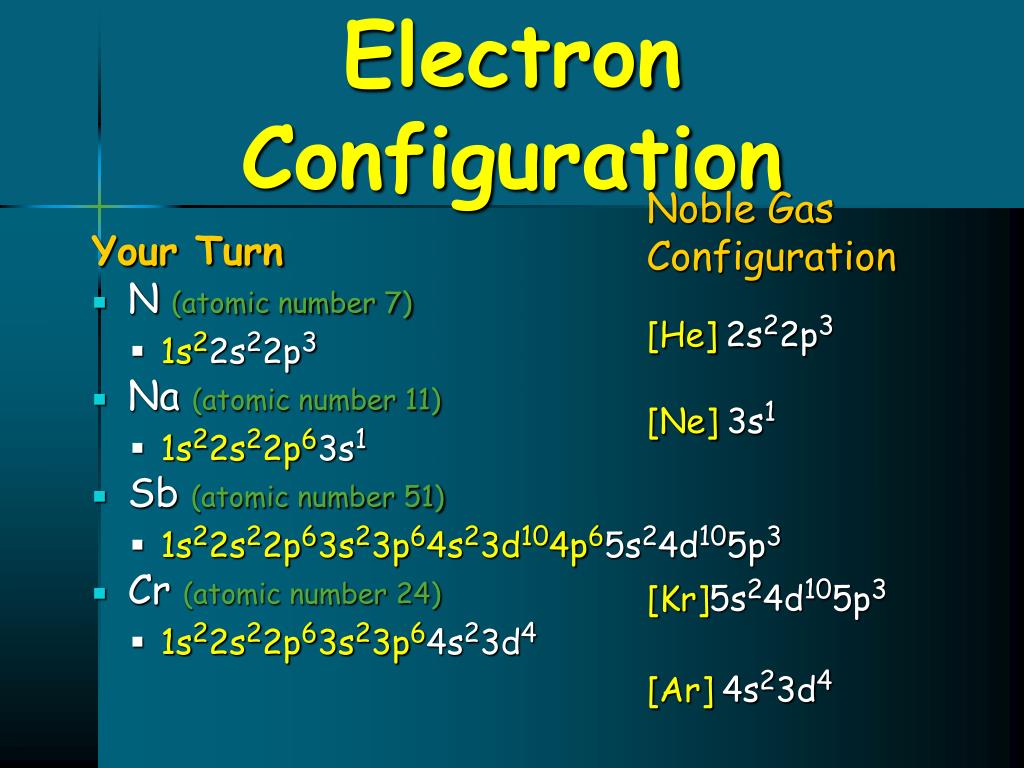
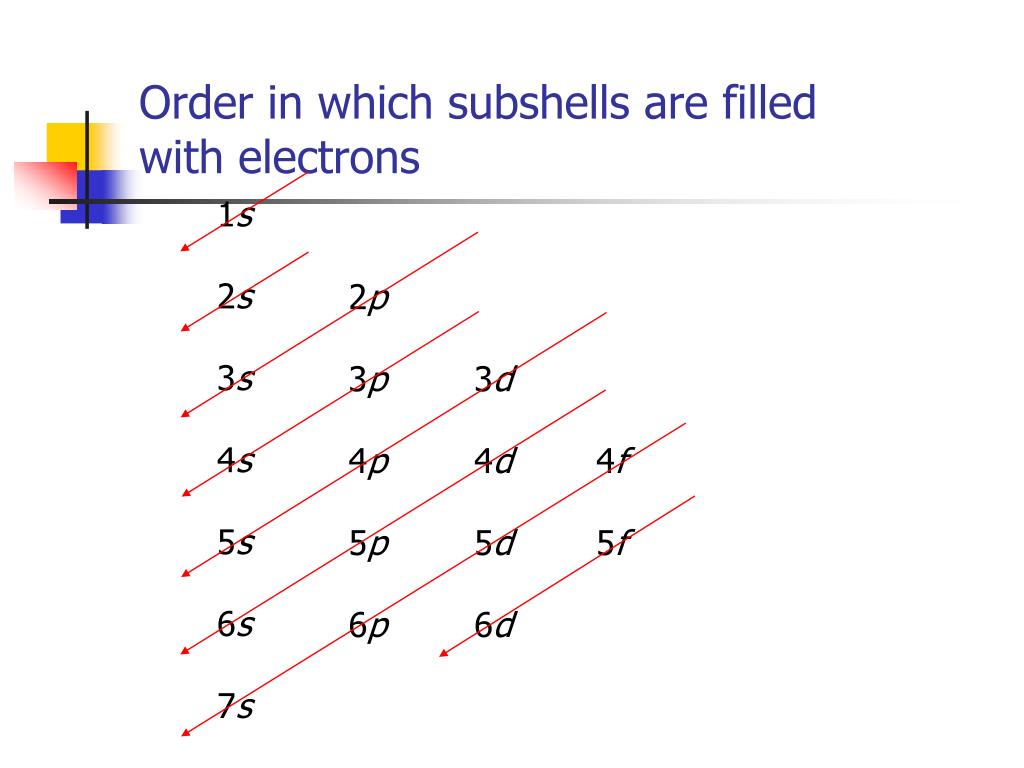
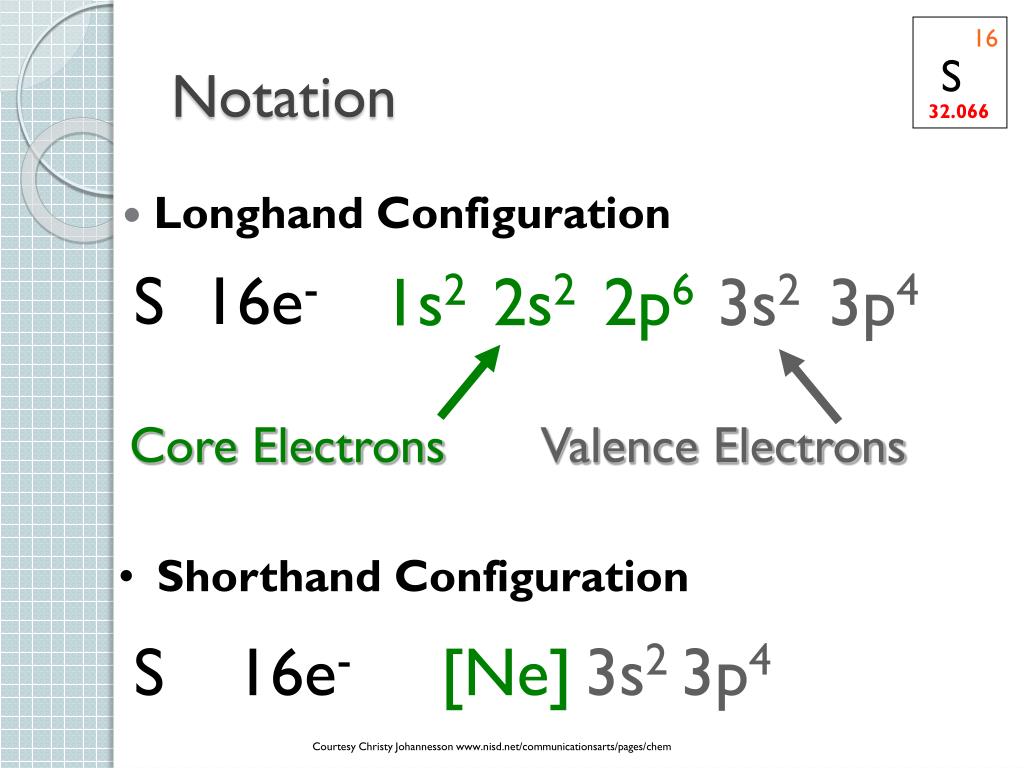
Ne Electron Configuration Long Form - It simply depicts the theory that the first two orbital. Periodic table of elements (wikipedia.org) for example, chlorine is in group 7,. The electron configuration of the ne can write as 1s 2 2s 2 2p 6. Since it is the outermost (valence) electrons. Web grayed out electron numbers indicate subshells filled to their maximum. Web the electron configuration of neon is [ he] 2s 2 2p 6, if the electron arrangement is through orbitals. Boron [he]2s 2 2p 1: Web neon (ne) electron configuration the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of. Web the principal quantum number n indicates the shell or energy level in which the electron is found. Web the group number that an element is in determines the number of its valence electrons. Boron [he]2s 2 2p 1: Web grayed out electron numbers indicate subshells filled to their maximum. The value of n can be set between 1 to n, where n is the value of. Web the electron configuration of neon is [ he] 2s 2 2p 6, if the electron arrangement is through orbitals. Web the principal quantum number n indicates. However, the first part of this long expression, 1s2 2s2 2s6, is the. Web what she was doing was writing the electron configuration of silicon. As 1s can only hold 2 electrons and the other next two electrons for. Web the group number that an element is in determines the number of its valence electrons. The electron configuration of the. As 1s can only hold 2 electrons and the other next two electrons for. Web the principal quantum number n indicates the shell or energy level in which the electron is found. Electron configuration can be done in two ways. For each atom the subshells are given first in concise form, then with all. Boron [he]2s 2 2p 1: In writing the electron configuration for nitrogen the first two. Web grayed out electron numbers indicate subshells filled to their maximum. Web how to write the electron configuration for nitrogen (n) nitrogen is the seventh element with a total of 7 electrons. However, the first part of this long expression, 1s2 2s2 2s6, is the. Web this page shows the. Web grayed out electron numbers indicate subshells filled to their maximum. Since it is the outermost (valence) electrons. As 1s can only hold 2 electrons and the other next two electrons for. Web the group number that an element is in determines the number of its valence electrons. Written in full this is 1s2 2s2 2s6 3s2 3p2. Electron configuration can be done in two ways. However, the first part of this long expression, 1s2 2s2 2s6, is the. The electron configuration of the ne can write as 1s 2 2s 2 2p 6. The value of n can be set between 1 to n, where n is the value of. Web all of the electrons in the. Web the group number that an element is in determines the number of its valence electrons. Web neon (ne) electron configuration the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of. However, the first part of this long expression, 1s2 2s2 2s6, is the. It simply depicts the theory. The value of n can be set between 1 to n, where n is the value of. Written in full this is 1s2 2s2 2s6 3s2 3p2. Electron configuration can be done in two ways. As 1s can only hold 2 electrons and the other next two electrons for. The electron configuration of the ne can write as 1s 2. Web neon (ne) electron configuration the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. As 1s can only hold 2 electrons and the other next two electrons for. Web nitrogen is a. Web neon (ne) electron configuration the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of. However, the first part of this long expression, 1s2 2s2 2s6, is the. Web when we write the electron configuration of n the first two electrons go in the 1s orbital. The electron configuration. Written in full this is 1s2 2s2 2s6 3s2 3p2. Web neon (ne) electron configuration the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web electron configurations are a simple way of writing down the locations of all of the electrons in an atom. Web grayed out electron numbers indicate subshells filled to their maximum. Electron configuration can be done in two ways. The value of n can be set between 1 to n, where n is the value of. Periodic table of elements (wikipedia.org) for example, chlorine is in group 7,. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. In writing the electron configuration for nitrogen the first two. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Web the electron configuration of neon is [ he] 2s 2 2p 6, if the electron arrangement is through orbitals. Web nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.the chemical symbol for nitrogen is n. Carbon [he]2s 2 2p 2:. Web what she was doing was writing the electron configuration of silicon. Since it is the outermost (valence) electrons. Web how to write the electron configuration for nitrogen (n) nitrogen is the seventh element with a total of 7 electrons. Web the principal quantum number n indicates the shell or energy level in which the electron is found. As 1s can only hold 2 electrons and the other next two electrons for.Be 1s22s2. Ne 1s22s22p6. Br 1s22s22p63s23p64s23d104p5. Dynamic
PPT Electron Configuration and the Periodic Table PowerPoint
List of Electron Configurations of Elements
PPT Electron Configuration Notation with Atomic Structure Review
Electron Configuration Of Neon In Excited State worksheet today
2.4 Electron Configurations Chemistry LibreTexts
CHEMISTRY ELECTRON CONFIGURATION LECTURE
PPT Electrons in Atoms PowerPoint Presentation, free download ID
Electron Configuration for Neon (Ne) Full Explanation
Electron Configuration Of Neon Long Form worksheet today
Related Post: