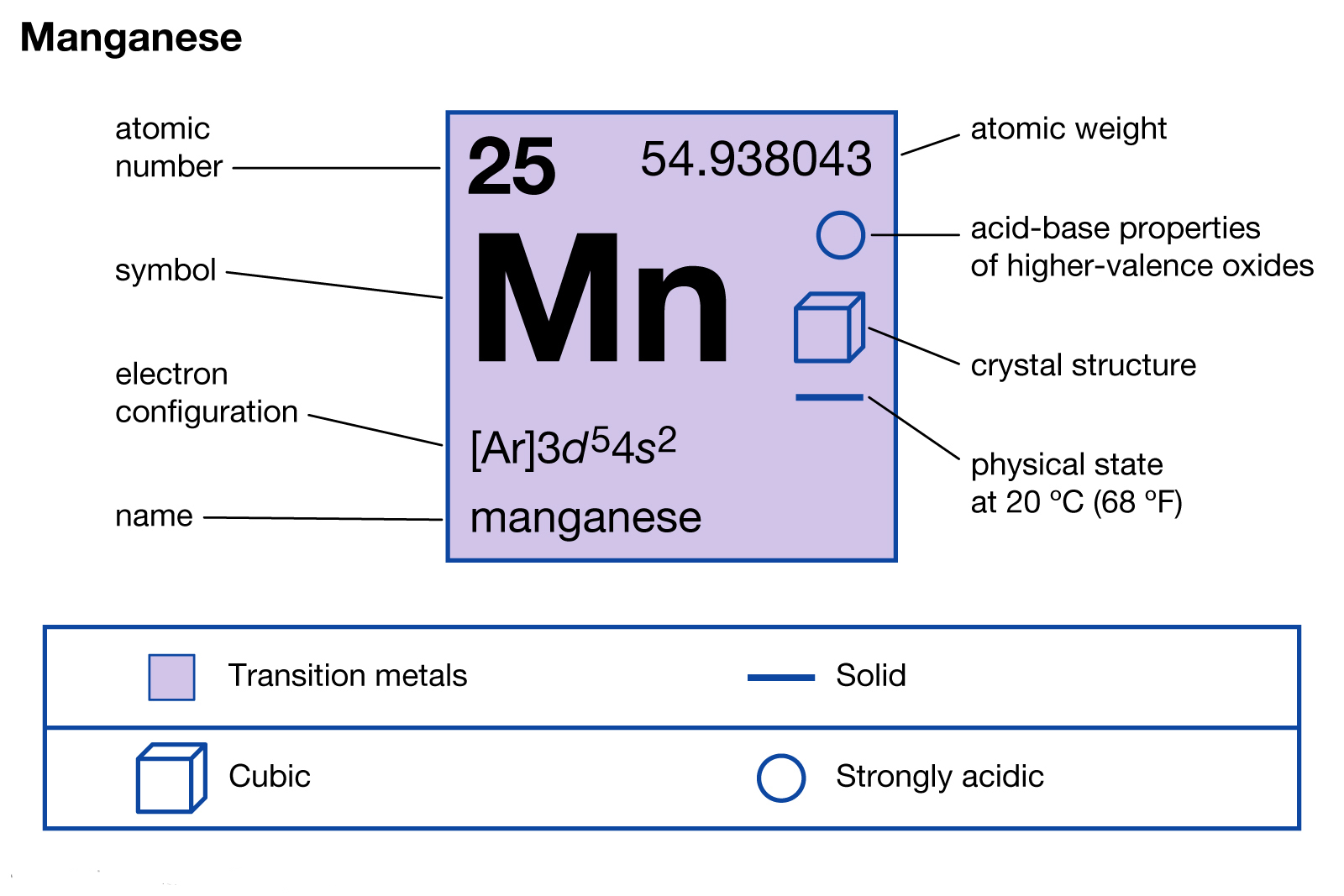
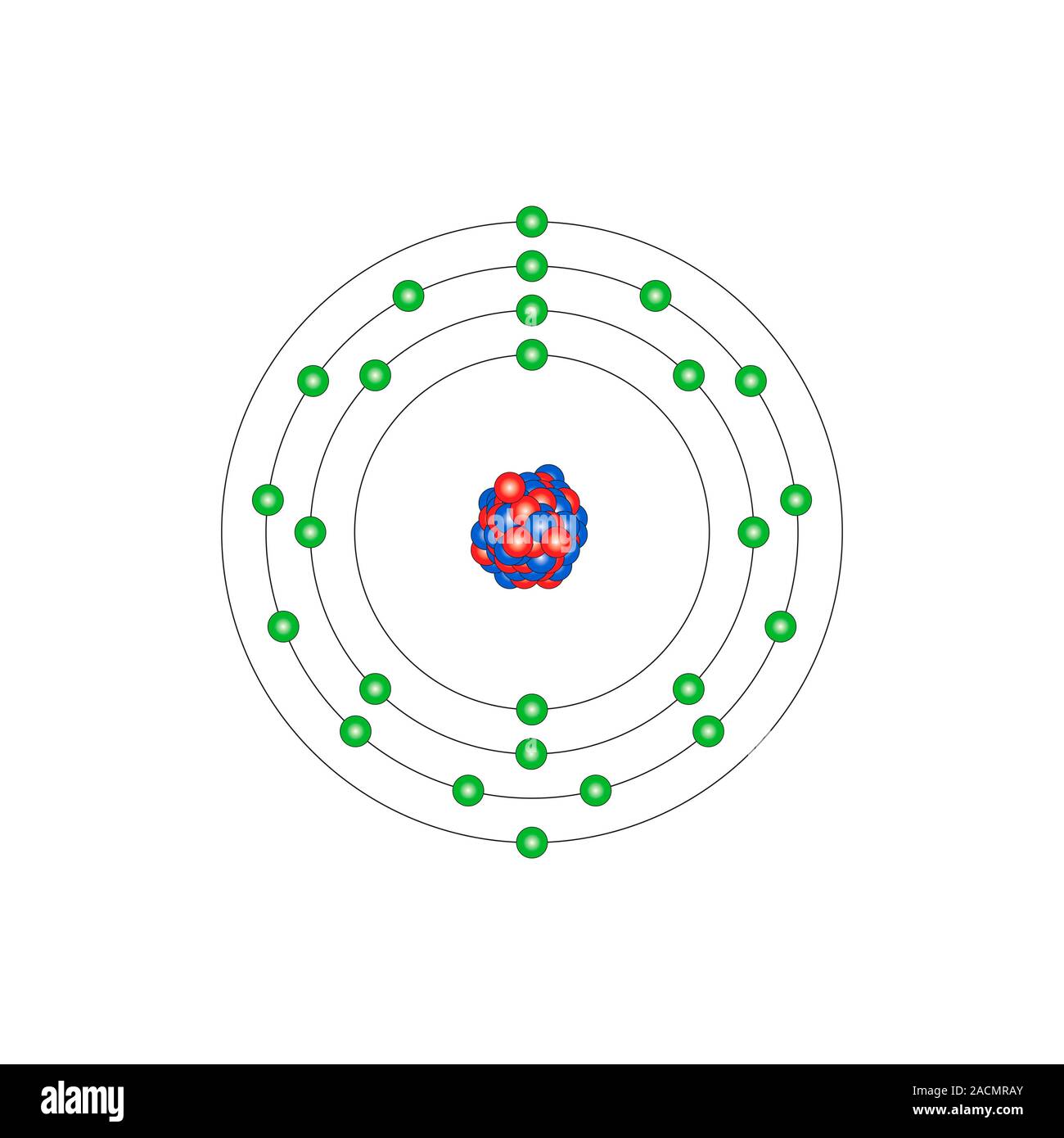
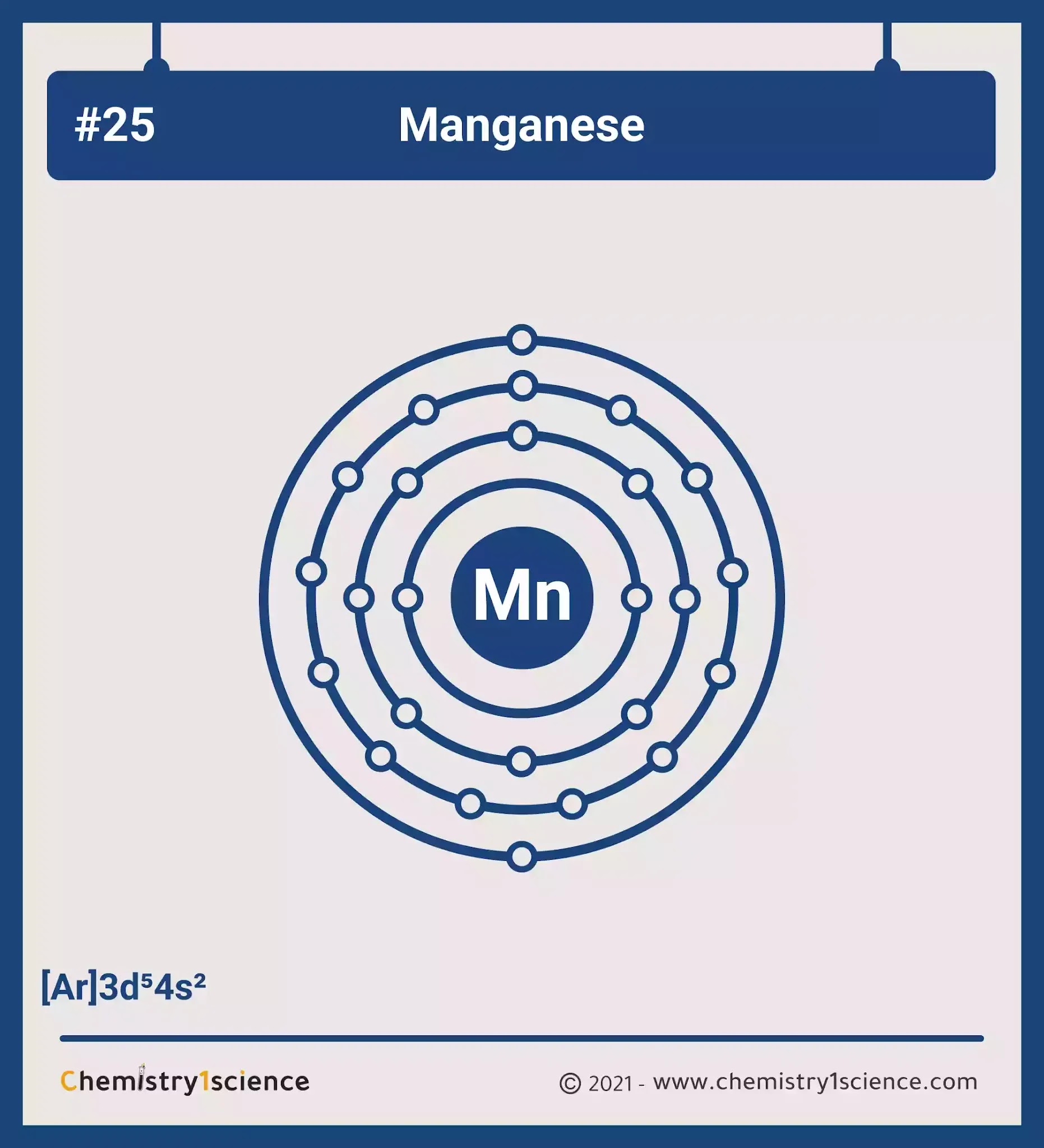
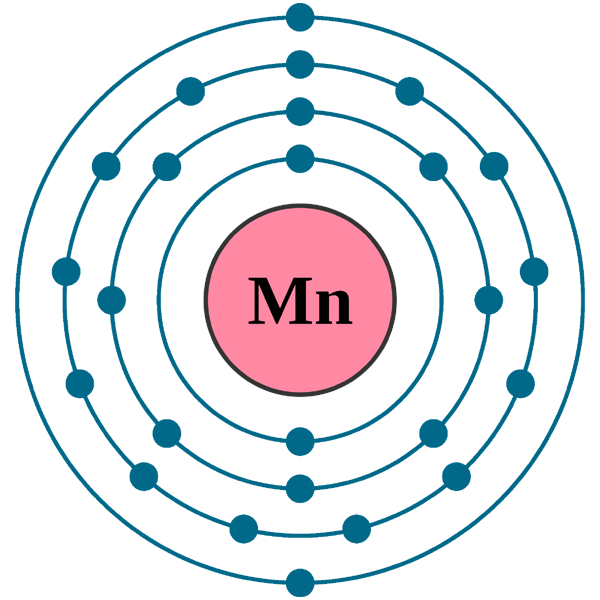
Manganese Electron Configuration Long Form
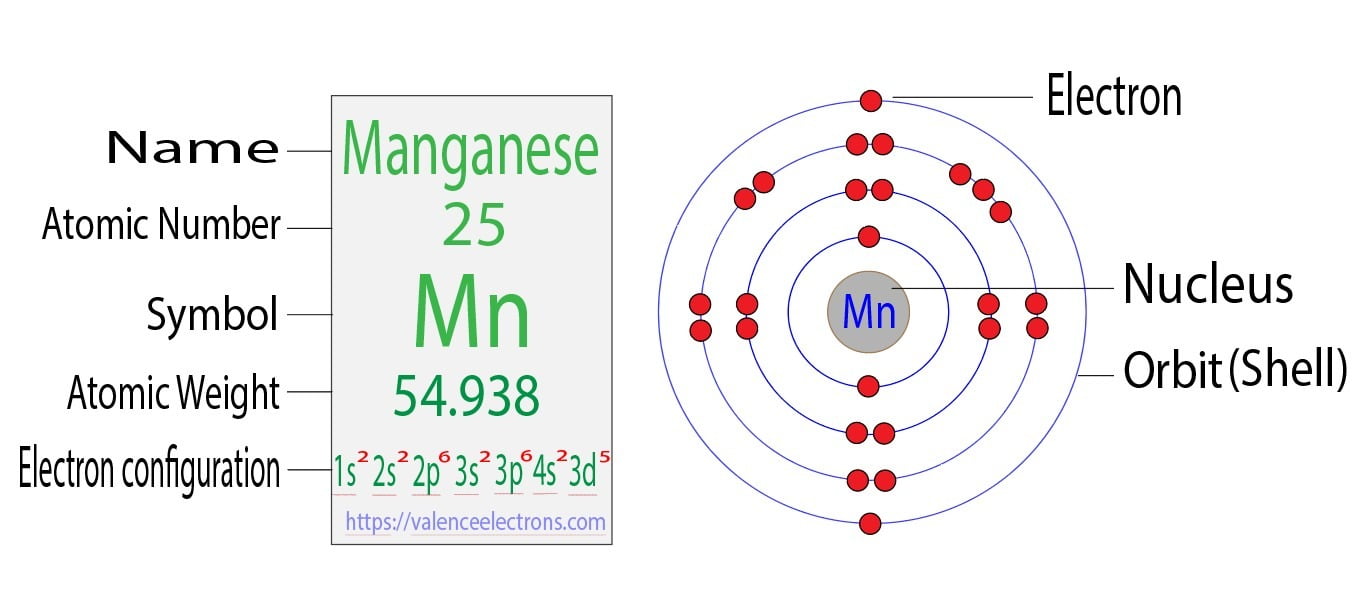
Manganese Electron Configuration Long Form - Simply use this information to obtain its electronic configuration. Element 25 of periodic table is manganese with atomic number 25, atomic weight 54.938049. Web manganese atoms have 25 electrons and the shell structure is 2.8.13.2. Web what is the electron configuration for manganese? Manganese atoms have to lose seven valence to attain noble gas configuration. Mn has an atomic number of 25. Web manganese, chemical element that is a silvery white, hard, brittle metal of group 7 in the periodic table. Manganese, symbol mn, has a body centered cubic structure and silver color. From the above information, we can say that manganese exhibits +2, +3, and +4 oxidation states. Possible oxidation states are +2,3,4,7. Simply use this information to obtain its electronic configuration. 1s 22s 22p 63s 23p 5. Manganese atoms have to lose seven valence to attain noble gas configuration. The electron configuration of manganese, atomic number 25, is 1s^22^22p^63s^23p^63d^54s^2. Binding energies of common chemical states: Web manganese, chemical element that is a silvery white, hard, brittle metal of group 7 in the periodic table. Binding energies of common chemical states: Oxidation states +7, +4, +3, +2: Today in this video, we will help you determine the electron configuration for the manganese element. What oxidation states can manganese take? Simply use this information to obtain its electronic configuration. First subshell contains 2 electrons. The diagram below represents the electron configuration as an orbital diagram. The electron configuration of manganese, atomic number 25, is 1s^22^22p^63s^23p^63d^54s^2. A vertical column in the periodic table. Web march 23, 2023 by jay. Manganese, symbol mn, has a body centered cubic structure and silver color. 656 views 11 months ago electron configuration. Schematic electronic configuration of manganese. Web 1.how can we write the manganese electron configuration long form? Electron configuration chart of all elements is mentioned in the table below. It was recognized as an element in 1774 by the swedish chemist carl wilhelm scheele. A horizontal row in the periodic table. Electron configuration and oxidation states of manganese. Web march 23, 2023 by jay. Web 1.how can we write the manganese electron configuration long form? The ground state electron configuration of ground state gaseous neutral manganese is [ ar ]. This page shows the electron configurations of the neutral gaseous atoms in their ground states. Its principal use is in the manufacture of alloy steel. A vertical column in the periodic table. The electron configuration of manganese is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5. The oxidation state of the element changes depending on the bond formation. Simply use this information to obtain its electronic configuration. Third subshell contains 13 electrons. Web manganese, chemical element that is a silvery white, hard, brittle metal of group. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table. Electron configuration of manganese is [ar] 3d5 4s2. Thus its electronic configuration is [ar]4s 23d 5 or. What oxidation states can manganese take? Electron configuration and oxidation states of manganese. Today in this video, we will help you determine the electron configuration for the manganese element. 4s2 and the term symbol is 6s5/2. Web the electron configuration of manganese ion (mn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3. Schematic electronic configuration of manganese. The oxidation state of the element changes depending on the bond formation. The metal is reactive chemically and decomposes slowly in cold water. Mn2+ often competes with mg2+ in biological systems. Oxidation states +7, +4, +3, +2: The diagram below represents the electron configuration as an orbital diagram. Web 1.how can we write the manganese electron configuration long form? Possible oxidation states are +2,3,4,7. Web march 23, 2023 by jay. The electron configuration of manganese is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5. Members of a group typically have similar properties and electron configurations in their outer shell. 1s 22s 22p 63s 23p 64s 24d 5. Web manganese, chemical element that is a silvery white, hard, brittle metal of group 7 in the periodic table. The ground state electron configuration of ground state gaseous neutral manganese is [ ar ]. Oxidation states +7, +4, +3, +2: [ar] 4s^2 3d^5 mn has an atomic number of 25. Web manganese is a chemical element with atomic number 25 which means there are 25 protons and 25 electrons in the atomic structure. Today in this video, we will help you determine the electron configuration for the manganese element. Binding energies of common chemical states: Manganese is a transition metal element. Manganese, symbol mn, has a body centered cubic structure and silver color. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Manganese improves rolling and forging qualities in steel, along with adding strength. Electron configuration of manganese is [ar] 3d5 4s2. From the above information, we can say that manganese exhibits +2, +3, and +4 oxidation states. Electron configuration and oxidation states of manganese.Manganese Electron Configuration Manganese Orbital Diagram Insight
Manganese Electron Configuration Dynamic Periodic Table of Elements
Manganese Electron Configuration Ground State / How many unpaired
Manganese (Mn). Diagram of the nuclear composition and electron
Manganese, atomic structure Stock Image C023/2488 Science Photo
Manganese Electron configuration Symbol Atomic Number Atomic
Manganese, atomic structure Stock Image C018/3706 Science Photo
Mn Electron Configuration Diagram / Electron Configurations Mn (z=25
Manganese (Mn). Diagram of the nuclear composition and electron
Electron Configuration for Magnesium and ion(Mg2+)
Related Post: