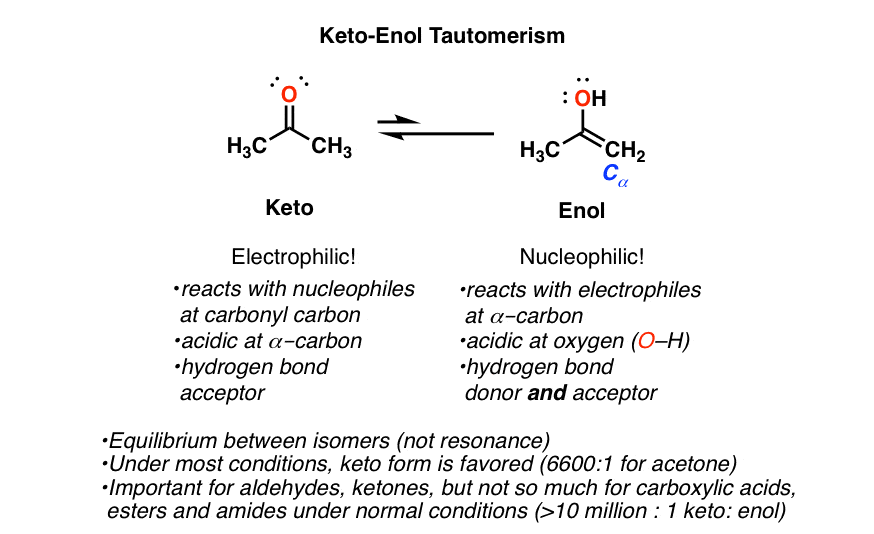
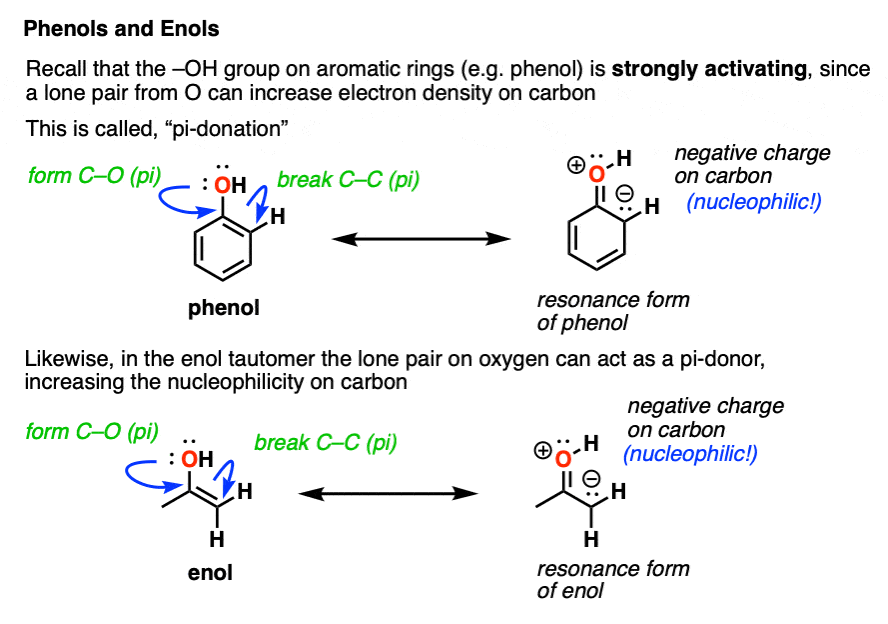
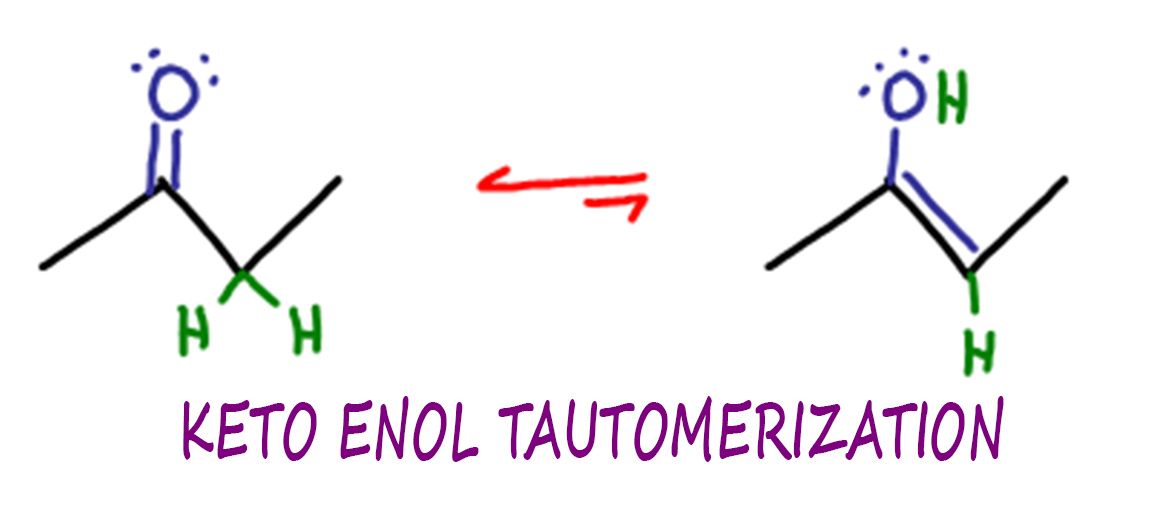
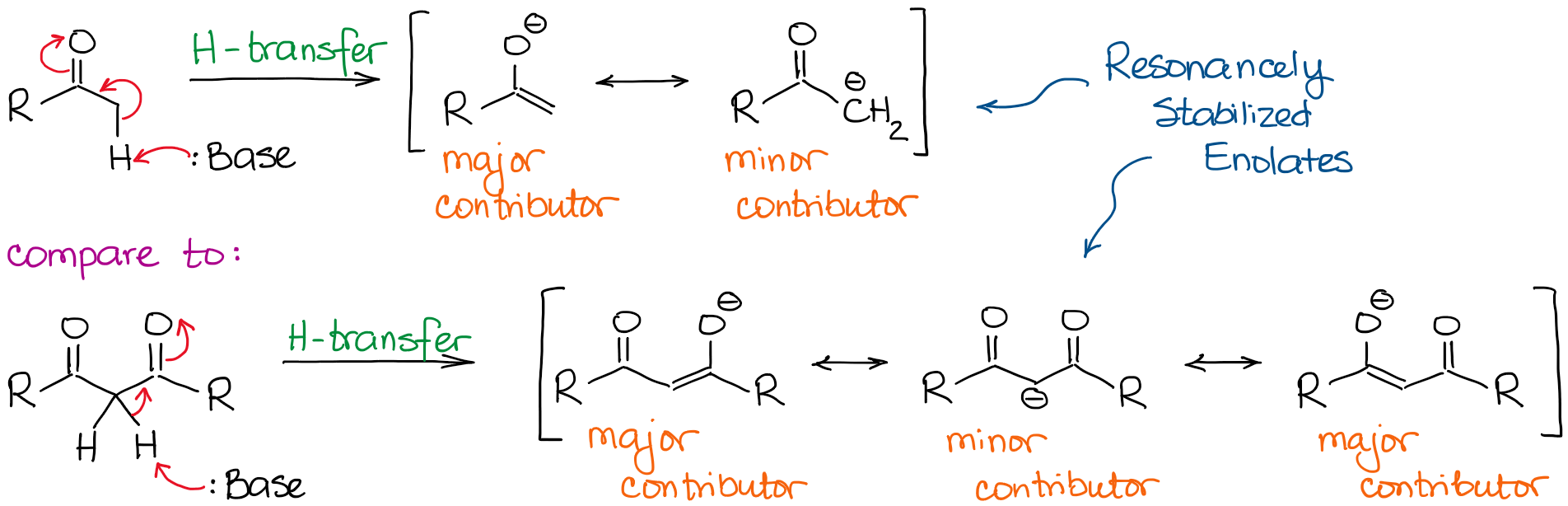
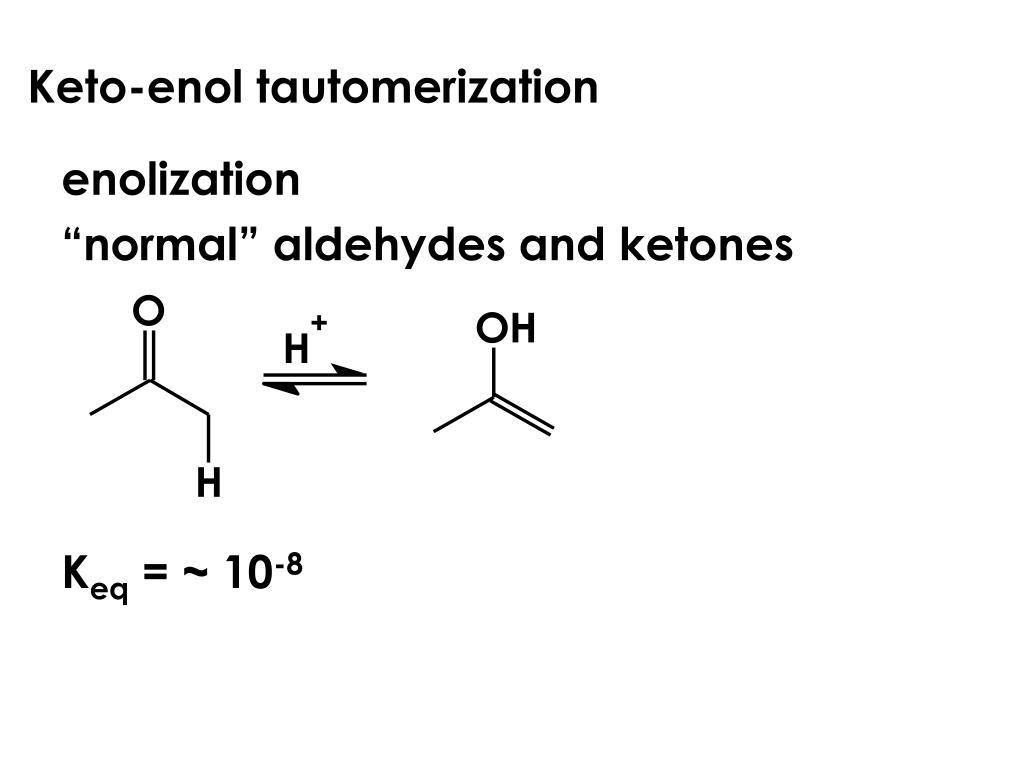
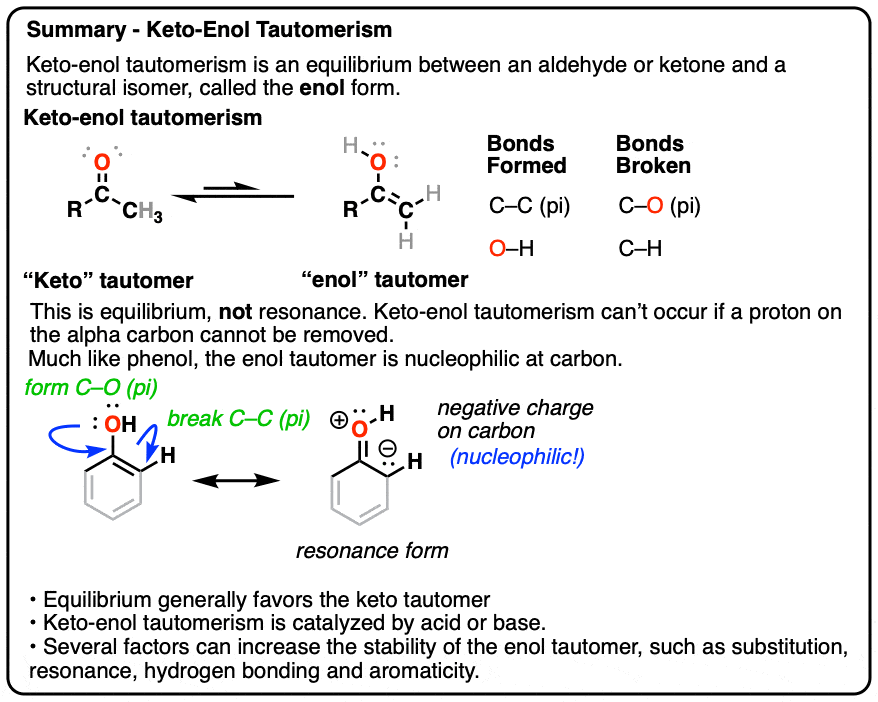
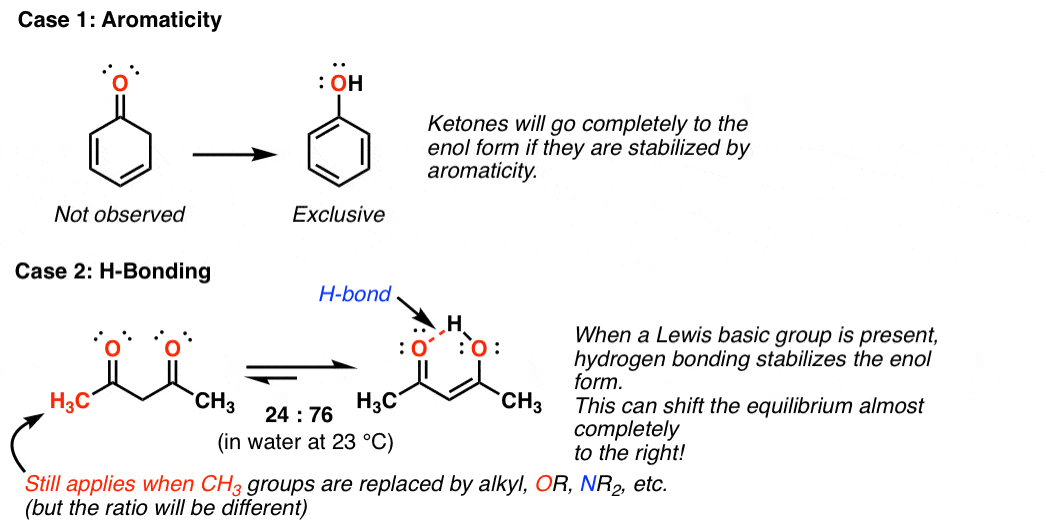
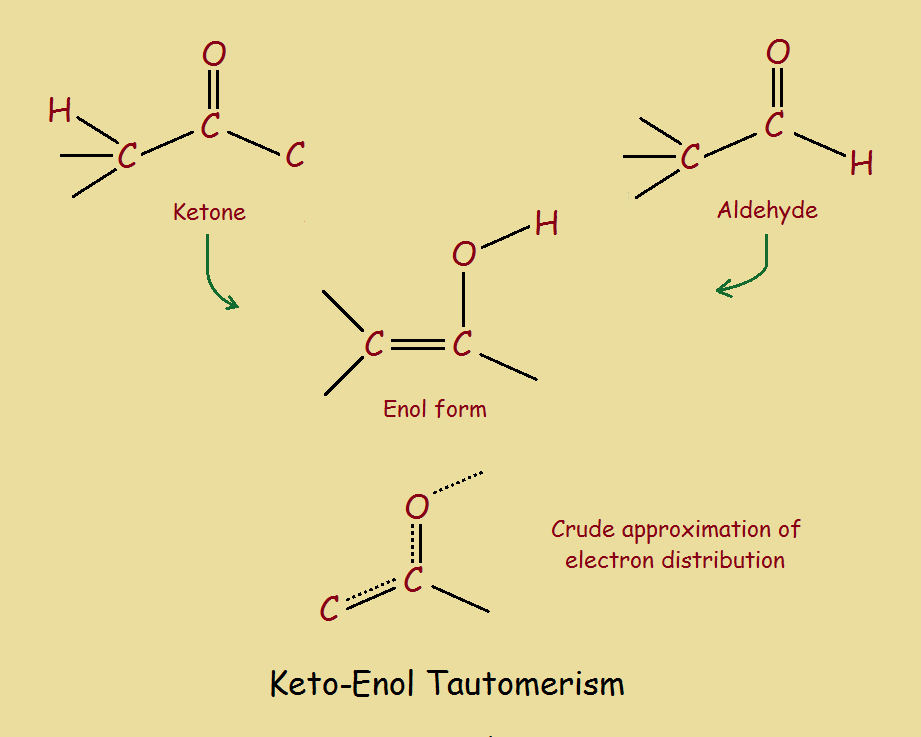
Keto Vs Enol Form
Keto Vs Enol Form - Web the implicit rationale for this observation is that the keto form is more polar than the enol form and hence is more stable in polar solvents. Web we would like to show you a description here but the site won’t allow us. Aldehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical ketones can have two or more. Web keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group. Web of course, such stabilization is not possible for the keto form. Web the keto form is normally favored since the c=o bond is stronger than a c=c bond. A short answer is none really 😊. When we refer to the enolization, we. Keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group. Tautomers are claimed to exist. Organic chemistry > unit 12. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (c=c−oh). Aldehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical ketones can have. However, the concept that the keto. Web we would like to show you a description here but the site won’t allow us. Keto form enol form h 3c ch3 oo h h c ch h h h h c ch h h h c ch h h. One can promote tautomerization to the enol form by reducing the amount of.. Keto form enol form h 3c ch3 oo h h c ch h h h h c ch h h h c ch h h. Web keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group. A short answer is none really 😊. However, the concept that the. When we refer to the enolization, we. Tautomers are claimed to exist. Web the keto form is normally favored since the c=o bond is stronger than a c=c bond. Aldehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical ketones can have two or more. Web we would like to show you a description here but the. One can promote tautomerization to the enol form by reducing the amount of. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (c=c−oh). An extreme example of the stabilization of an. Aldehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical ketones can have two or more. Web with this information, it is possible to calculate that about 10 per cent of the enol is present in the rapidly exchanging mixture at 110 degrees, by virtue of the relative position of the. When we refer to the enolization,. Web enol or keto? Web keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group. However, the concept that the keto. Web the implicit rationale for this observation is that the keto form is more polar than the enol form and hence is more stable in polar solvents.. An extreme example of the stabilization of an enol by electron delocalization is benzenol (phenol),. Keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group. Organic chemistry > unit 12. However, the concept that the keto. Tautomers are claimed to exist. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (c=c−oh). Web with this information, it is possible to calculate that about 10 per cent of the enol is present in the. Keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group. Aldehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical ketones can have two or more. Keto form enol form h 3c ch3 oo h h c ch h h h h c ch h. Web keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group. An extreme example of the stabilization of an enol by electron delocalization is benzenol (phenol),. A short answer is none really 😊. Web of course, such stabilization is not possible for the keto form. Web with this information, it is possible to calculate that about 10 per cent of the enol is present in the rapidly exchanging mixture at 110 degrees, by virtue of the relative position of the. Keto form enol form h 3c ch3 oo h h c ch h h h h c ch h h h c ch h h. Organic chemistry > unit 12. One can promote tautomerization to the enol form by reducing the amount of. However, the concept that the keto. Web the implicit rationale for this observation is that the keto form is more polar than the enol form and hence is more stable in polar solvents. When we refer to the enolization, we. Keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group. Web enol or keto? Aldehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical ketones can have two or more. Web we would like to show you a description here but the site won’t allow us. Web the keto form is normally favored since the c=o bond is stronger than a c=c bond. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (c=c−oh). Tautomers are claimed to exist.KetoEnol Tautomerism Key Points Master Organic Chemistry
KetoEnol Tautomerism Key Points Master Organic Chemistry
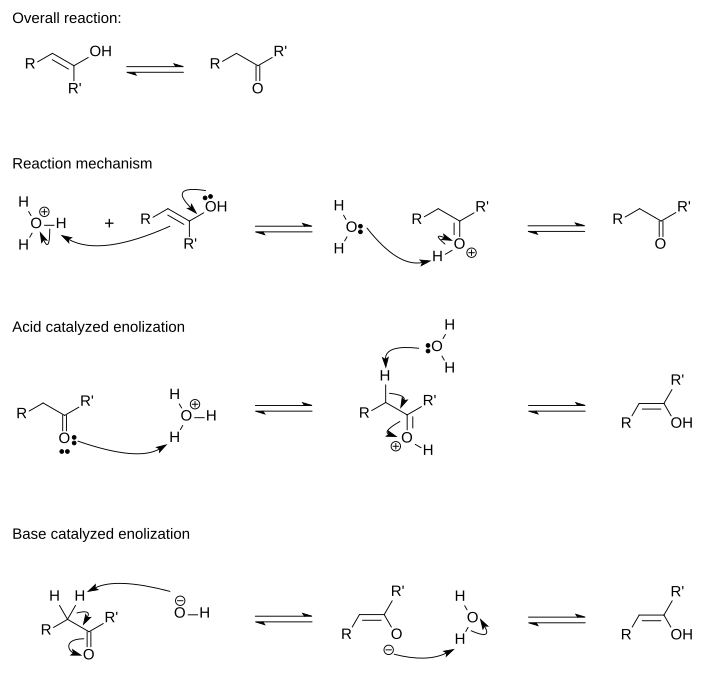
Keto Enol Tautomerization Reaction and Mechanism in Acid and Base
Enolization & KetoEnol Tautomerism — Organic Chemistry Tutor
PPT Ketoenol tautomerization PowerPoint Presentation, free download
KetoEnol Tautomerism Key Points Master Organic Chemistry
KetoEnol Tautomerism Key Points Master Organic Chemistry
Keto Enol Tautomerism What Is It and Why Is It Important?
Ketoenol tautomerism Wikipedia
KetoEnol Tautomerism Key Points Master Organic Chemistry
Related Post: