Iron Reacts With Oxygen To Form Iron Iii Oxide
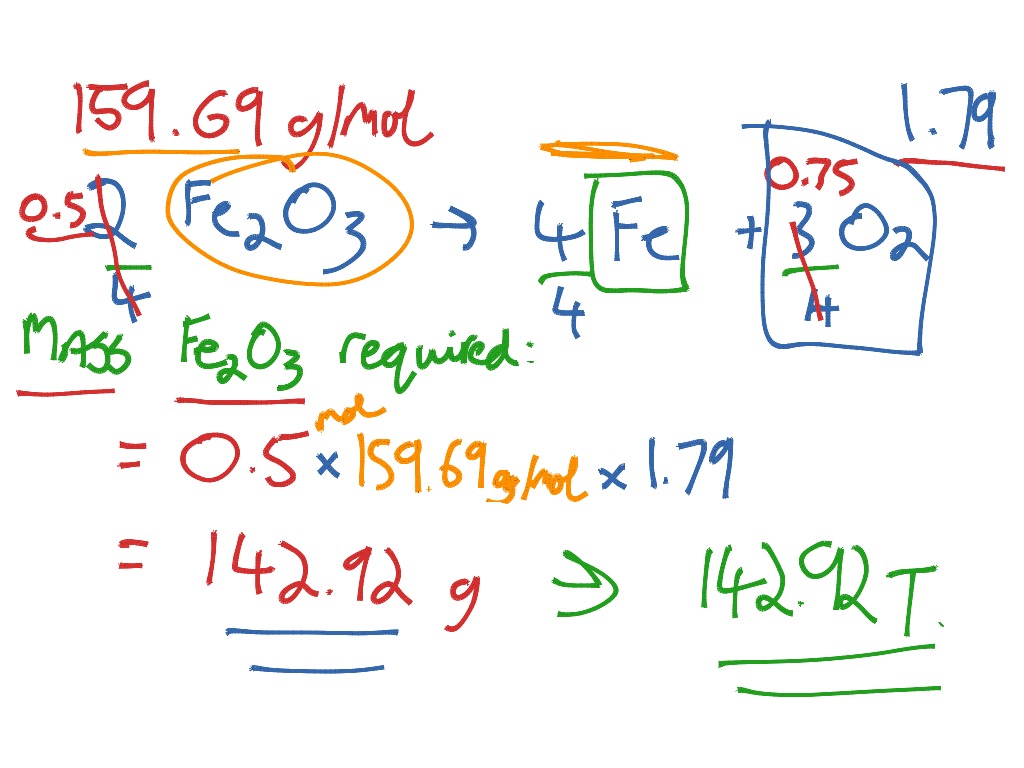
Iron Reacts With Oxygen To Form Iron Iii Oxide - Web iron reacts with oxygen to produce iron (iii) oxide. Web science chemistry iron reacts with oxygen to form iron (iii) oxide: This will balance the oxygens. Web we will put a e in front of the molecular oxygen and a 2 in front of the iron (iii) oxide. Iron + water + oxygen → hydrated iron. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. How many grams of product will be formed from 5.00g of fe? 3fe + 2o₂ = feo • fe₂o₃. Web chemistry questions and answers. Fe + 3o2 ==> 2fe2o3. O, 16.00 g/mol) a) 13.1. Web when iron is left outside, overtime it reacts with oxygen to form iron oxide or rust. Write the balanced chemical equation. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of iron (fe) (fe) is. Web chemistry questions and answers. Calculate the theoretical yield of iron (iii) oxide ( fe2o3 ) and the reaction produces 8.36 g of fe2o3. Web when iron is left outside, overtime it reacts with oxygen to form iron oxide or rust. 4 fe (s) + 3 0, (g) 2 fe,0, (s) suppose 17.2 g of iron (fe) is reacted with 16.6 g of oxygen. Web. Iron reacts with oxygen to form iron (iii) oxide. Web science chemistry iron reacts with oxygen to form iron (iii) oxide: Iron oxides are products of reaction. Web iron reacts with oxygen to form iron (iii) oxide (fe2o3). Web chemistry questions and answers. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. This will balance the oxygens. This is what a cutting torch does. Web science chemistry iron reacts with oxygen to form iron (iii) oxide: O, 16.00 g/mol) a) 13.1. Web chemistry questions and answers. Write balanced equations for the chemical reactions described. How many grams of product will be formed from 5.00g of fe? When two smaller substances combine to form. This is what a cutting torch does. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Iron reacts with oxygen to form iron (iii) oxide. Web iron reacts with oxygen at high temperatures to form iron (iii) oxide. Iron reacts with oxygen to form iron (iii) oxide. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose. Web when iron is left outside, overtime it reacts with oxygen to form iron oxide or rust. 4 fe (s) + 3 o 2 (g) ==> 2 fe 2 o 3 (s) iron in its usual bulk solid form will only burn when in pure oxygen with when a great deal of heat is supplied. Iron (iii) oxide reacts with. Web iron reacts with oxygen to form iron (iii) oxide (fe2o3). Web iron reacts with oxygen at high temperatures to form iron (iii) oxide. Web we will put a e in front of the molecular oxygen and a 2 in front of the iron (iii) oxide. Enter a balanced chemical equation for this reaction. How many grams of product will. Iron oxides are products of reaction. 4 fe (s) + 3 0, (g) 2 fe,0, (s) suppose 17.2 g of iron (fe) is reacted with 16.6 g of oxygen. Web iron reacts with oxygen to form iron (iii) oxide (fe2o3). 4 f e ( s) + 3 o 2 ( g) → 2 f e 2 o 3 ( s). 4fe(s)+3o2(g) 2fe2o3(s)4fe(s)+3o2(g) 2fe2o3(s) suppose 11.6 g11.6 g of iron. Web iron reacts with oxygen to form iron (iii) oxide (fe2o3). Web when iron is left outside, overtime it reacts with oxygen to form iron oxide or rust. Write the balanced chemical equation. This will balance the oxygens. Calculate the theoretical yield of iron (iii) oxide ( fe2o3 ) and the reaction produces 8.36 g of fe2o3. Here is the word equation for the reaction: Web science chemistry iron reacts with oxygen at high temperatures to form iron (ii) oxide. Web when iron is left outside, overtime it reacts with oxygen to form iron oxide or rust. Iron reacts with oxygen to form iron (iii) oxide. Web iron reacts with oxygen to produce iron (iii) oxide. Web 3fe + 2o₂ = fe₃o₄, or. Iron reacts with oxygen to form iron (iii) oxide. If you leave iron inside, this also happens because there is as much. 4 f e ( s) + 3 o 2 ( g) → 2 f e 2 o 3 ( s) if 6.4 moles of fe react with excess o 2, how many moles of f e 2 o 3 can be formed?. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of iron (fe) (fe) is. 4 fe (s) + 3 0, (g) 2 fe,0, (s) suppose 17.2 g of iron (fe) is reacted with 16.6 g of oxygen. 4fe(s)+3o2(g) 2fe2o3(s)4fe(s)+3o2(g) 2fe2o3(s) suppose 11.6 g11.6 g of iron. Write balanced equations for the chemical reactions described. Web it can be prepared in the laboratory by electrolyzing a solution of sodium bicarbonate, an inert electrolyte, with an iron anode: This is what a cutting torch does. Web iron reacts with oxygen at high temperatures to form iron (iii) oxide. Web we will put a e in front of the molecular oxygen and a 2 in front of the iron (iii) oxide. Fe + 3o2 ==> 2fe2o3.PPT The Law of Conservation of Matter PowerPoint Presentation, free
How to Write the Formula for Iron (III) Oxide YouTube
of Iron(III)oxide Science, Chemistry, Stoichiometry
Solved 6. Iron reacts with oxygen gas to form iron (III)
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID3552860
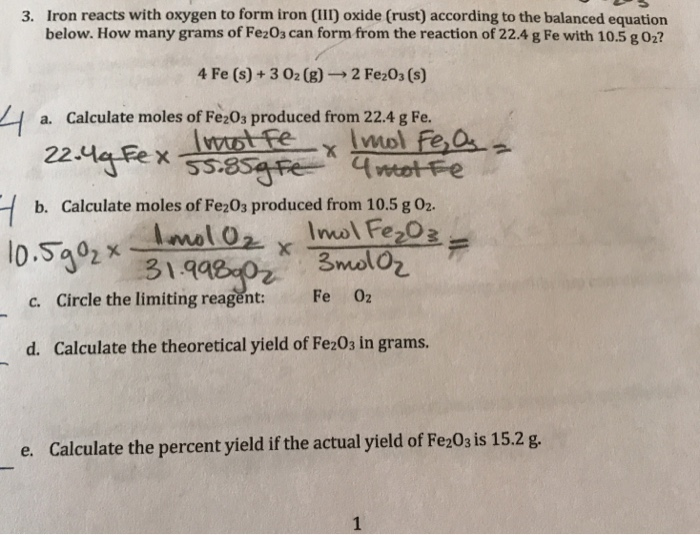
Solved 3. Iron reacts with oxygen to form iron (III) oxide
SOLVEDIron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a
Easy Steps for Balancing Chemical Equations
when iron rusts, solid iron reacts with gaseous oxygen to form solid
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379
Related Post:



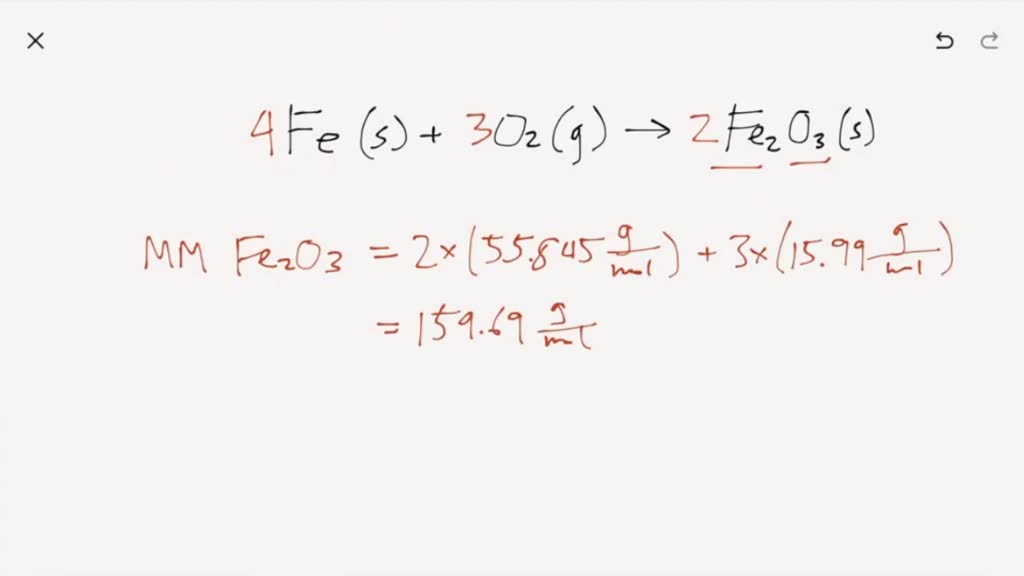
:max_bytes(150000):strip_icc()/BalanceEquations1-56a132765f9b58b7d0bcf535.png)

