In What Form Can An Ionic Compound Conduct Electricity
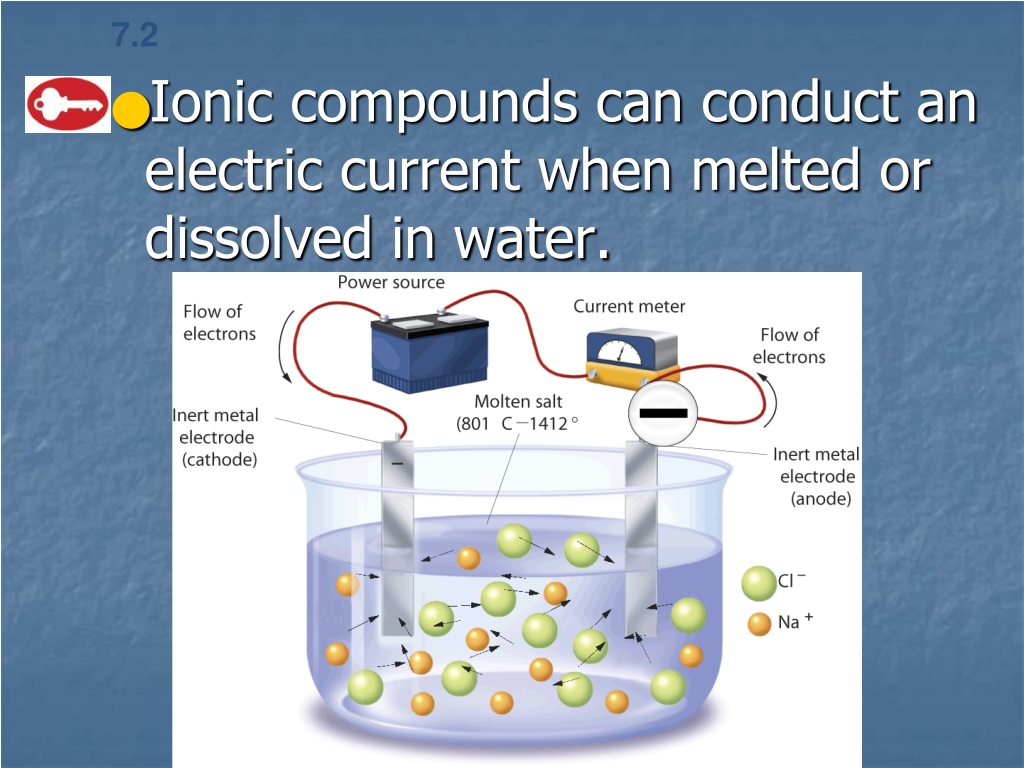
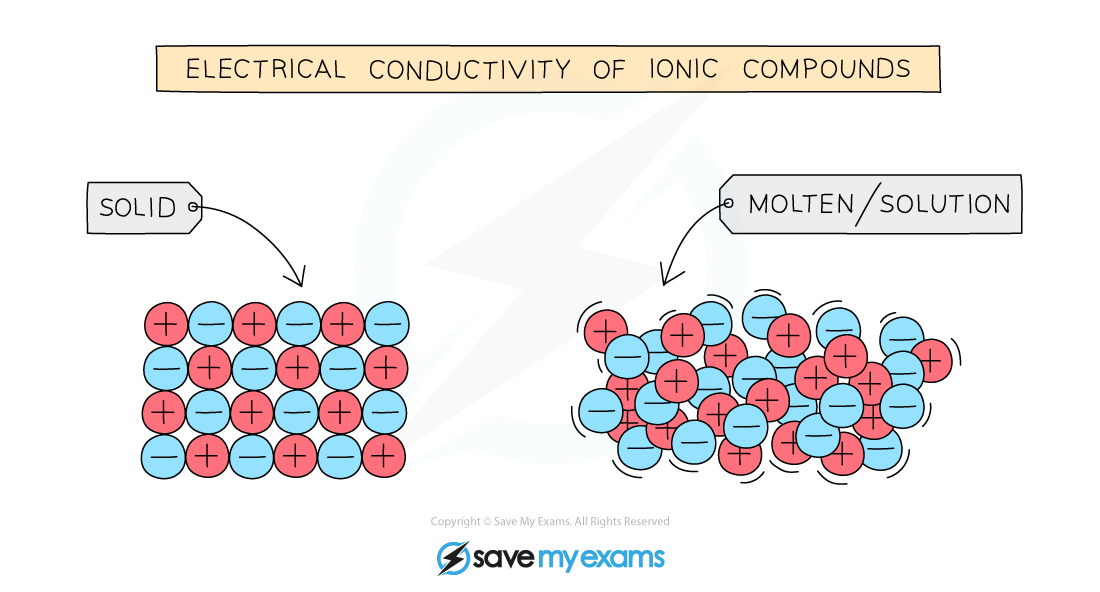
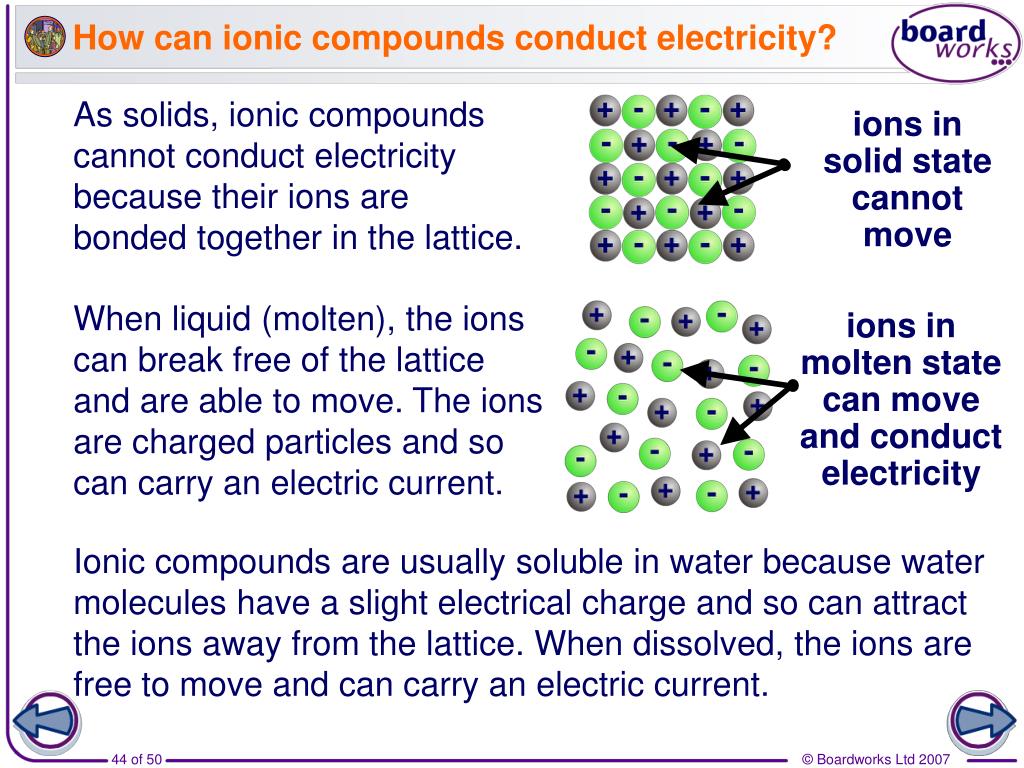
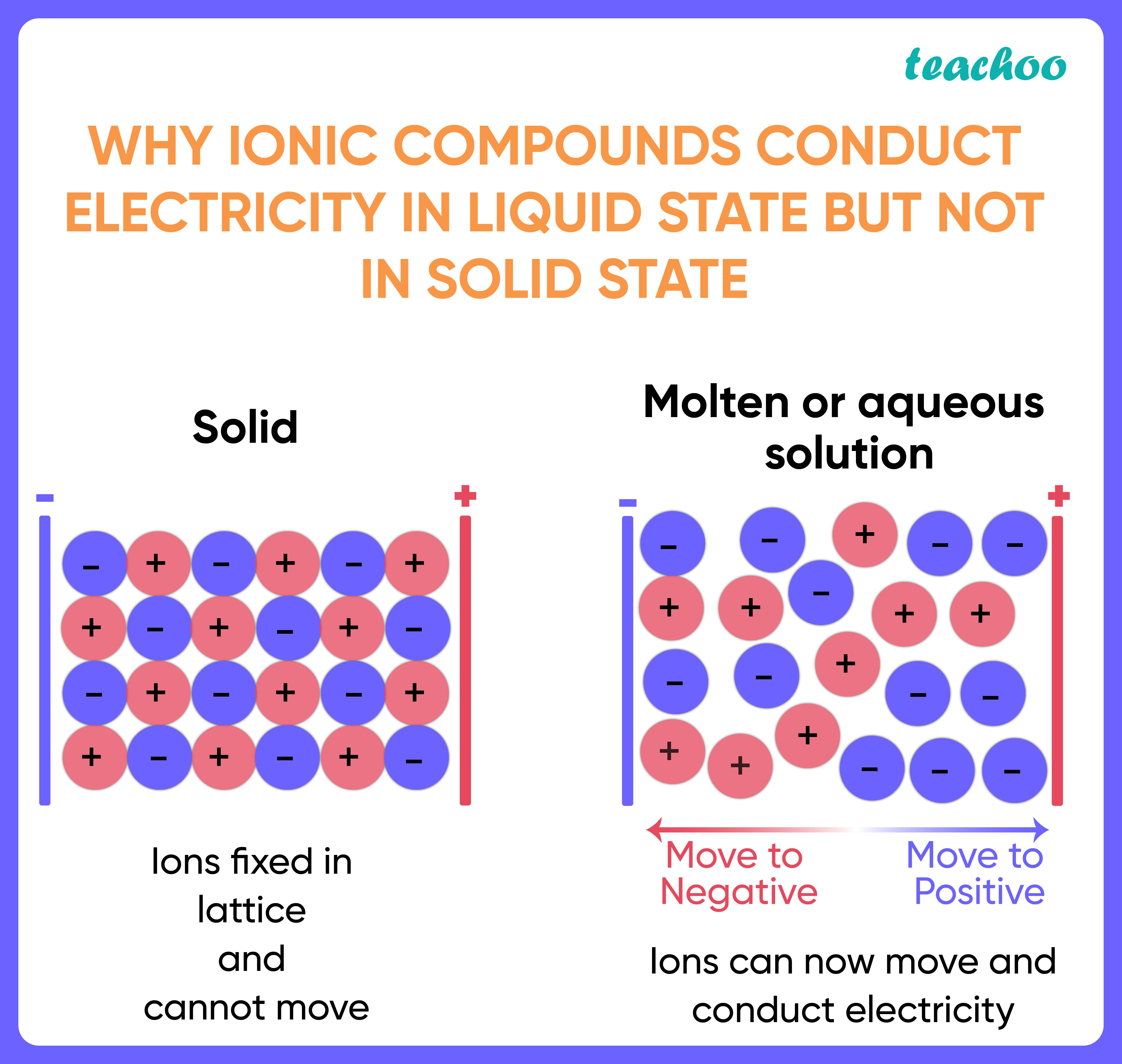
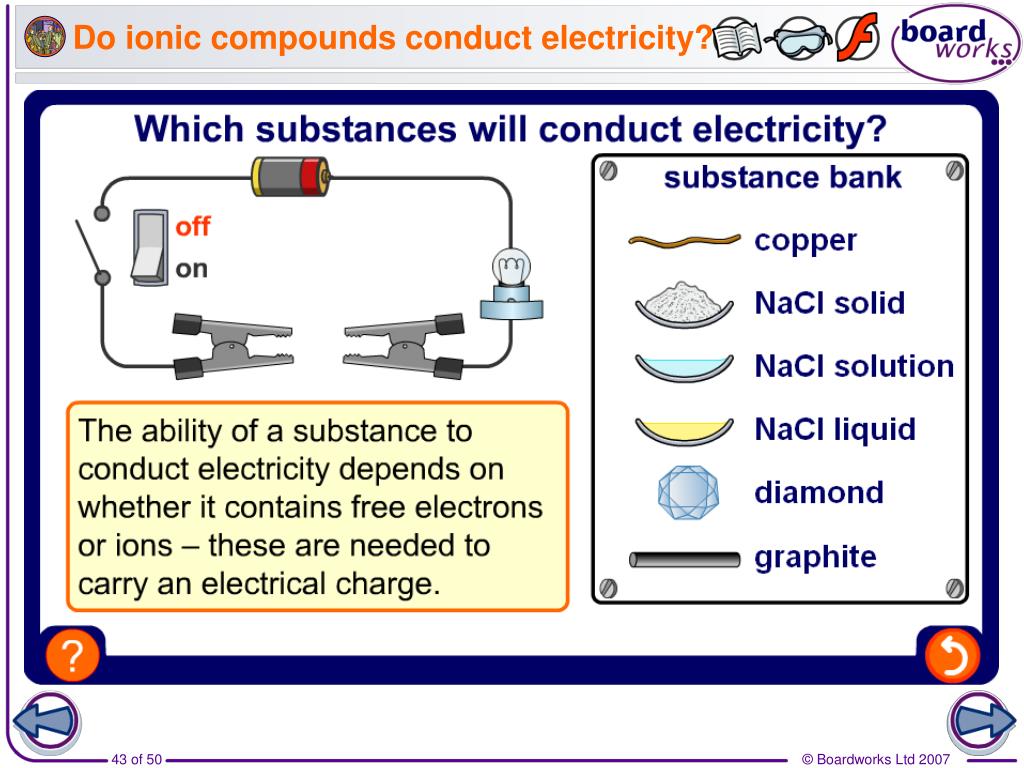
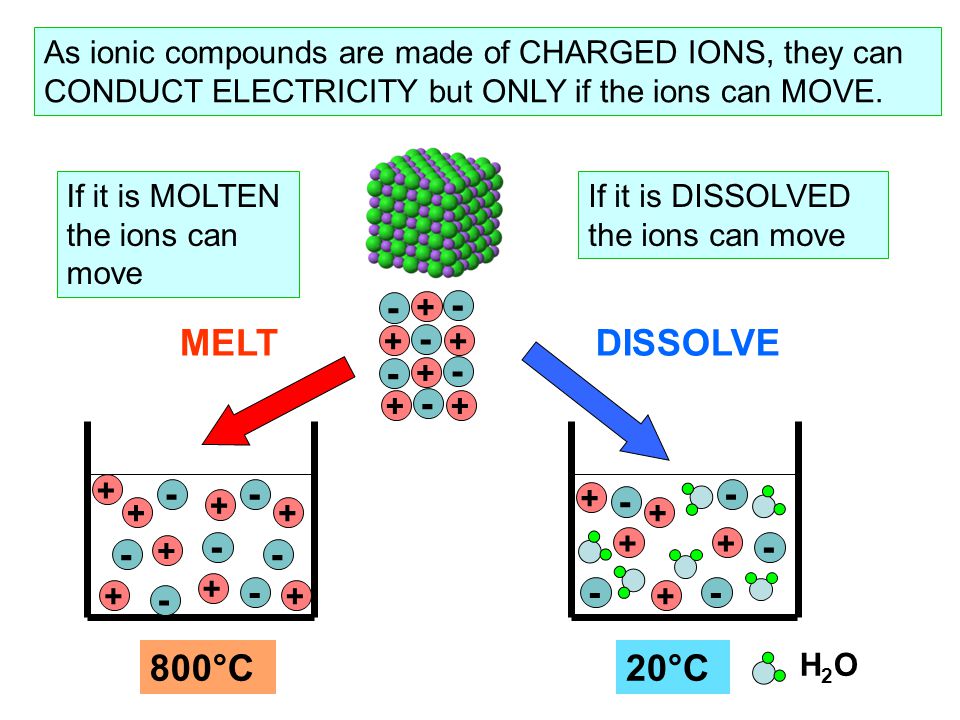
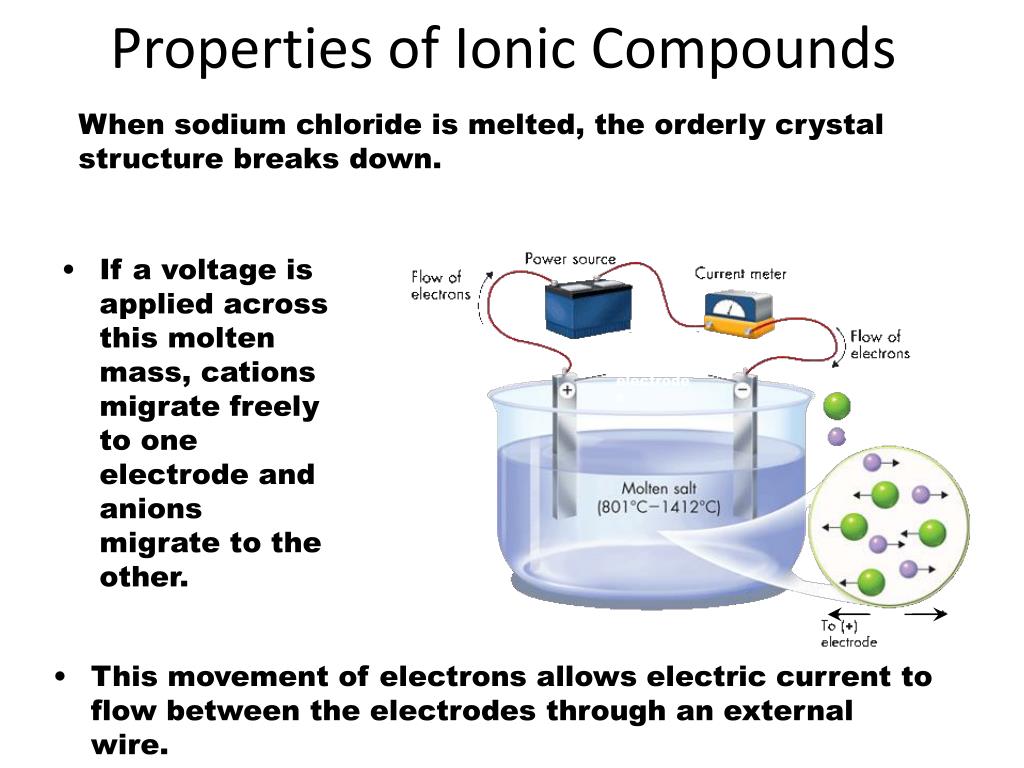
In What Form Can An Ionic Compound Conduct Electricity - Web we would like to show you a description here but the site won’t allow us. Also why don't they conduct in solid form and only in liquid or gaseous forms? Web solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. An ionic compound can be identified by its. Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ad browse & discover thousands of science book titles, for less. Whereas in the molten state, ionic compounds conduct electricity as. Web ionic compounds can conduct electricity when dissolved in water, since the ions dissociate, current can travel through the solution. Web ionic compounds cannot conduct electricity in the solid state because their ions are held in fixed positions and cannot move. Web electricity conduction in ionic compounds: Ionic compounds usually exist in solid state and are regarded as high. Web ionic compounds can conduct electricity when dissolved in water, since the ions dissociate, current can travel through the solution. An ionic compound can be identified by its. Web ionic compounds can conduct electricity when they are in molten form. In an ionic solution, the \(\ce{a^+}\) ions migrate. If electrons can move around freely (as in the delocalized bonds of metals), then. Or do they use electrons? Web ionic compounds can conduct electricity when dissolved in water, since the ions dissociate, current can travel through the solution. They conduct electricity only if their ions are free to move — in the molten state or in. Also why don't. Web solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. That charge can be positive or negative, but that doesn't matter for whether or not. Ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot. Web ionic compounds do not conduct electricity when they are solid. Ionic. If electrons can move around freely (as in the delocalized bonds of metals), then. Web in short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the oppositely charged electrode. Ad includes 5e lesson plans, reading material, quiz games, diy activities & more. In an aqueous state or molten state, ions are.. Ionic compounds can conduct electricity when they are. Web ( 1 vote) upvote flag jpogle a year ago something is polar if it has an electrical charge. In an ionic solution, the \(\ce{a^+}\) ions migrate toward. Ionic compounds are made from metallic elements bonded to nonmetallic elements. Web ionic compounds are conductors of electricity when they are in a molten. Ionic compounds usually exist in solid state and are regarded as high. Or do they use electrons? Key fact ionic compounds conduct electricity when. Web solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot. Web ionic compounds cannot conduct electricity in the solid state because their ions are held in fixed positions and cannot move. Key fact ionic compounds conduct electricity when. They conduct electricity only if their ions are free to move — in the molten state or in. Ad browse & discover thousands of science book titles, for less. In an aqueous. In an aqueous state or molten state, ions are. Includes a lesson plan, activity, worksheet, quiz, video, reading & more. Or do they use electrons? Ionic compounds can conduct electricity when they are. Ad includes 5e lesson plans, reading material, quiz games, diy activities & more. They conduct electricity only if their ions are free to move — in the molten state or in. Ionic compounds usually exist in solid state and are regarded as high. Web the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. Web in solid form, an ionic compound is not electrically conductive. Web ionic compounds can conduct electricity when dissolved in water, since the ions dissociate, current can travel through the solution. If electrons can move around freely (as in the delocalized bonds of metals), then. Metals give up electrons and therefore become positive charged. In an aqueous state or molten state, ions are. Web in solid form, an ionic compound is. Ad includes 5e lesson plans, reading material, quiz games, diy activities & more. Includes a lesson plan, activity, worksheet, quiz, video, reading & more. That charge can be positive or negative, but that doesn't matter for whether or not. Ionic compounds usually exist in solid state and are regarded as high. Web solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Web ionic compounds cannot conduct electricity in the solid state because their ions are held in fixed positions and cannot move. Web electricity conduction in ionic compounds: Ad browse & discover thousands of science book titles, for less. Ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot. Also why don't they conduct in solid form and only in liquid or gaseous forms? Web this is because both processes make their ions free to move from place to place. Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Web we would like to show you a description here but the site won’t allow us. Ionic compounds can conduct electricity when they are. Web the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. Key fact ionic compounds conduct electricity when. They conduct electricity only if their ions are free to move — in the molten state or in. Metals give up electrons and therefore become positive charged. An ionic compound can be identified by its. Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles).PPT Chapter 7 Ionic and Metallic Bonding PowerPoint Presentation
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure
PPT What are bonds? PowerPoint Presentation, free download ID5980343
The table shown below gives information about four Chemistry MCQ
Do Ionic Bonds Conduct Electricity
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Conductivity Of Ionic Compounds slidesharedocs
PPT Chapter 7 PowerPoint Presentation, free download ID2380065
PPT Chapter 9 PowerPoint Presentation, free download ID3330042
Ionic Bonding Presentation Chemistry
Related Post:

.PNG)