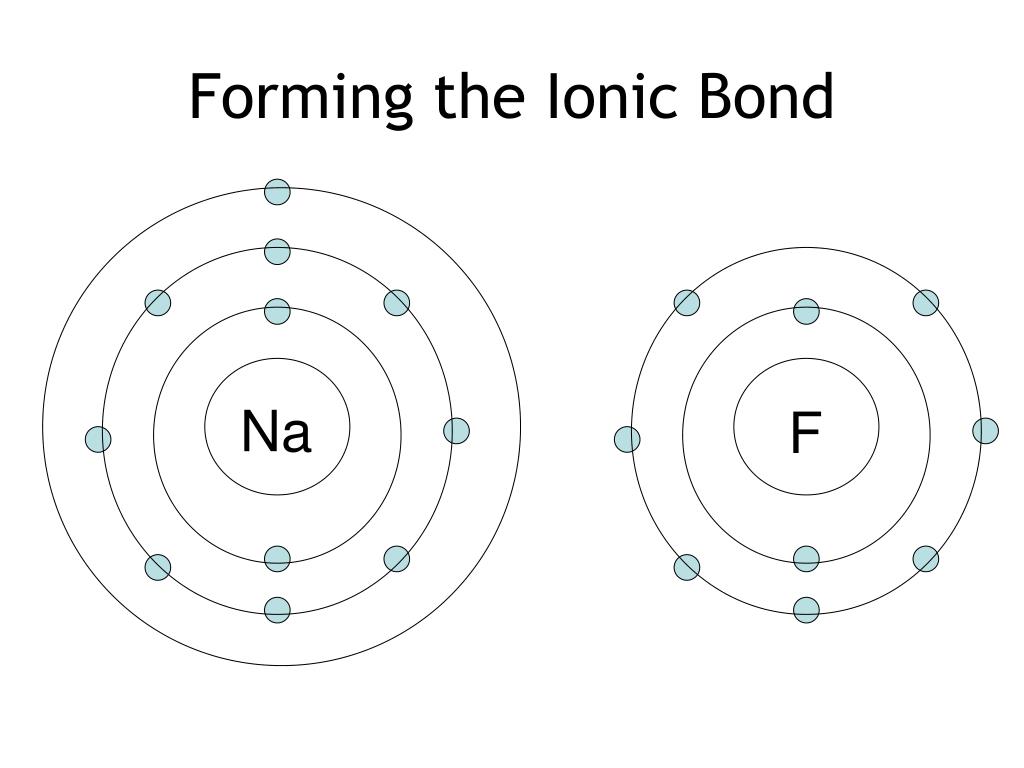
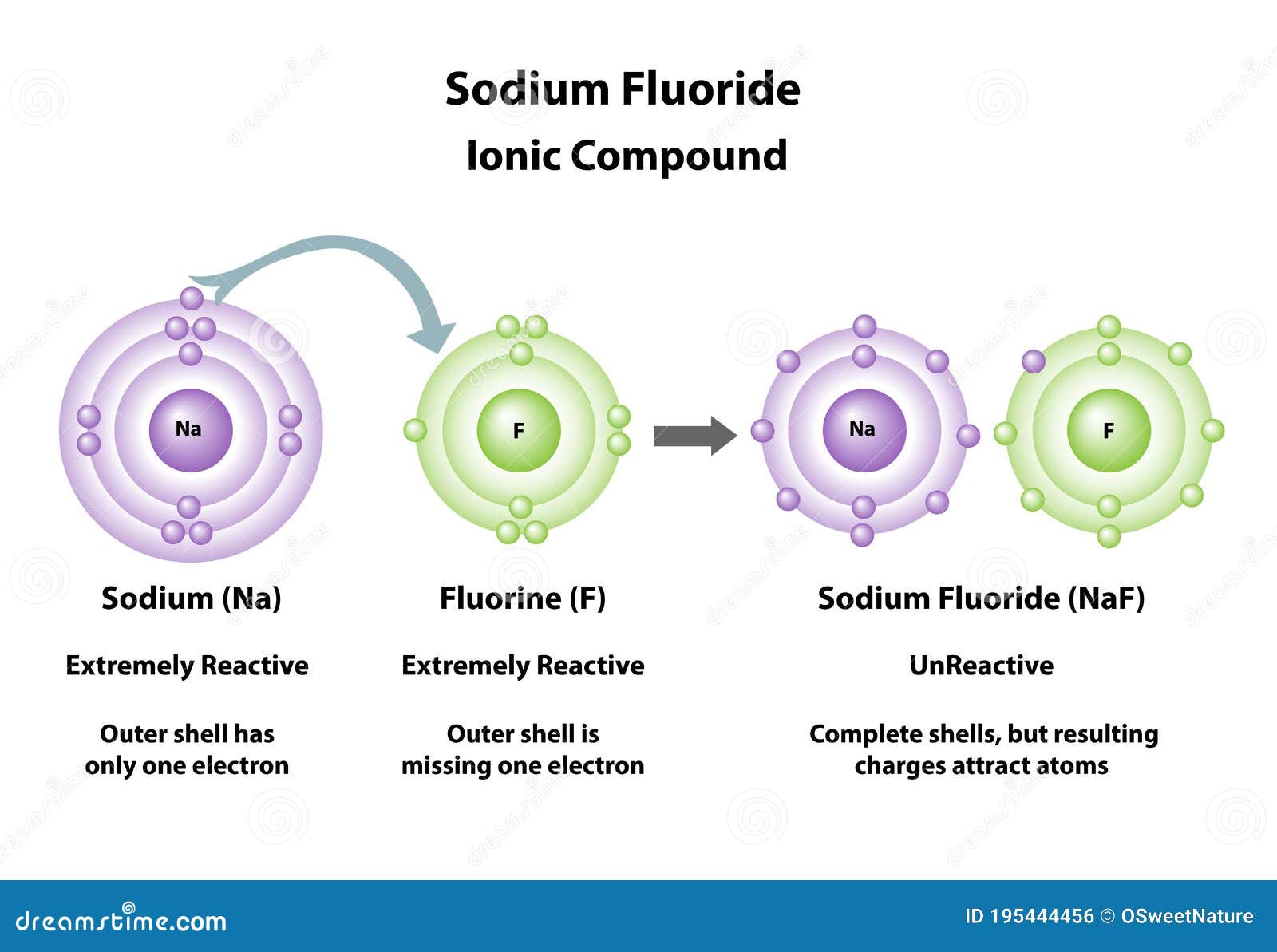
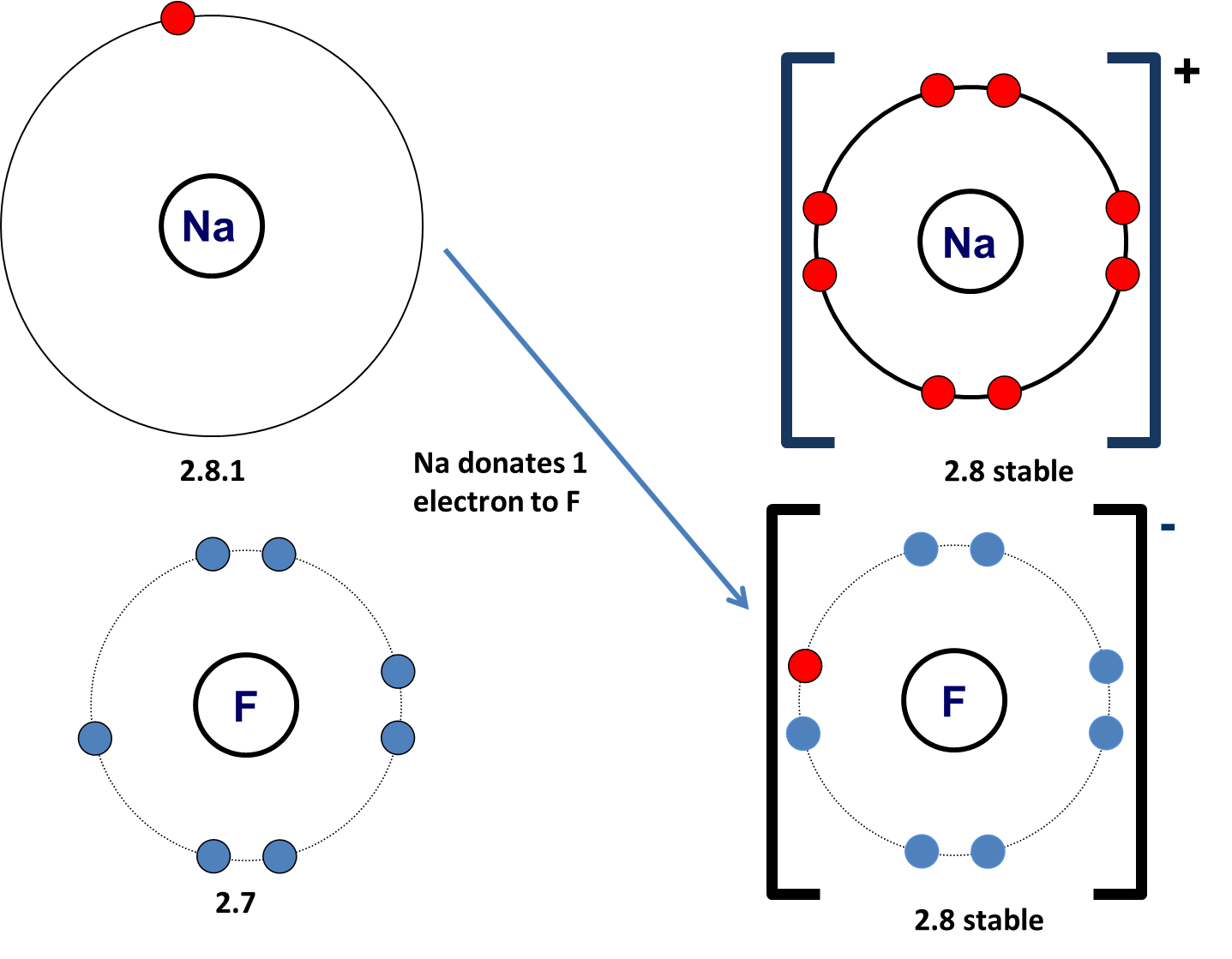
Does Sodium And Fluorine Form An Ionic Compound
Does Sodium And Fluorine Form An Ionic Compound - Web sodium and fluorine will form an ionic compound named sodium fluoride with the formula naf. Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. Web the charge of the metal ion is determined from the formula of the compound and the charge of the anion. How to identify an ionic compound: We know that n a. Examples of fluorides include sodium fluoride and. (flourine is not a chemical name!) examples of metals are sodium, magnesium, lanthanum, and. Correct options are b) and c) sodium has 11 electrons in it, which can be written as 2,8,1. In covalent bonding, the electrons that form the. Web any metallic element will form an ionic compound with fluorine. Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. In doing so, cations are formed. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their electrons to achieve a stable electron configuration. Web 4 years ago. What is the name of the. Examples of fluorides include sodium fluoride and. The electron from a sodium atom transfers to a chlorine atom. Web do fluorine and sodium form an ionic compound? Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their electrons to achieve a stable electron configuration. Web sodium. Thus we see that sodium has 1 electron in its outermost. The formula for an ionic compound follows several conventions. An atom of another element (usually nonmetal) with greater electron affinity accepts one or more electrons to attain a stable electron configuration, and after accep… For example, consider binary ionic compounds of iron and. Web any metallic element will form. An ionic compound exhibits some signature recognizable properties, such. Examples of fluorides include sodium fluoride and. Web thus n a f is. Correct options are b) and c) sodium has 11 electrons in it, which can be written as 2,8,1. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low,. Web the charge of the metal ion is determined from the formula of the compound and the charge of the anion. The formula for an ionic compound follows several conventions. How to identify an ionic compound: Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. In covalent bonding, the electrons that form the. Correct options are b) and c) sodium has 11 electrons in it, which can be written as 2,8,1. The electron from a sodium atom transfers to a chlorine atom. How to identify an ionic compound: Web thus n a f is. Thus we see that sodium has 1 electron in its outermost. Web sodium and fluorine will form an ionic compound named sodium fluoride with the formula naf. Web do fluorine and sodium form an ionic compound? Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. (flourine is not a chemical name!) examples of metals are sodium, magnesium, lanthanum, and. Web the outer electron from a sodium. Web 4 years ago. Can fluorine and sodium for a ionic compound? Web the charge of the metal ion is determined from the formula of the compound and the charge of the anion. What is the name of the ionic compound made from one sodium and. Examples of fluorides include sodium fluoride and. In doing so, cations are formed. H+ is just a proton, so no electrons would be present. Web sodium and fluorine will form an ionic compound named sodium fluoride with the formula naf. Web in this case, naf is an ionic compound, meaning that the sodium atom donates an electron to the fluoride atom, resulting in the formation of a. Web any metallic element will form an ionic compound with fluorine. Web in this case, naf is an ionic compound, meaning that the sodium atom donates an electron to the fluoride atom, resulting in the formation of a positively. How to identify an ionic compound: We know that n a. Thus we see that sodium has 1 electron in its. In covalent bonding, the electrons that form the. The formula for an ionic compound follows several conventions. We know that n a. Web the outer electron from a sodium atom transfers to the outer shell of a chlorine atom. In doing so, cations are formed. Examples of fluorides include sodium fluoride and. Can fluorine and sodium for a ionic compound? Correct options are b) and c) sodium has 11 electrons in it, which can be written as 2,8,1. Web do fluorine and sodium form an ionic compound? Web in this case, naf is an ionic compound, meaning that the sodium atom donates an electron to the fluoride atom, resulting in the formation of a positively. For example, consider binary ionic compounds of iron and. The electron from a sodium atom transfers to a chlorine atom. Web any metallic element will form an ionic compound with fluorine. Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their electrons to achieve a stable electron configuration. An ionic compound exhibits some signature recognizable properties, such. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least. Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. Web thus n a f is. Web 4 years ago.PPT Ionic Bonding L.O. PowerPoint Presentation, free download ID
Ionic Bonding Presentation Chemistry
What are Ionic Compounds and how they are formed?
⚗️What is the correct way to represent the ionic compound sodium
PPT IONIC BONDING PowerPoint Presentation, free download ID1785078
Ionic Bonding Elements are the simplest substances There
PPT Chapter 19— CHEMICAL BONDS PowerPoint Presentation, free download
Sodium Fluoride Ionic Compound Created Stock Vector Illustration of
Structure and bonding GCSE chemistry
What is the correct way to represent the ionic compound sodium fluoride
Related Post:
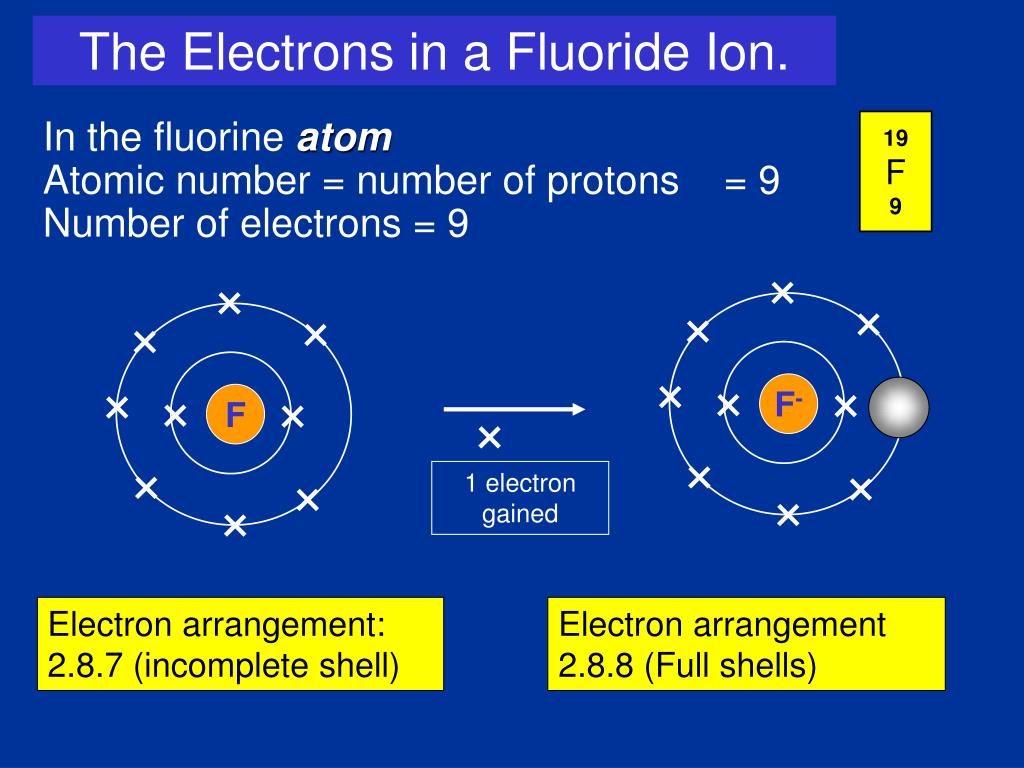
.PNG)