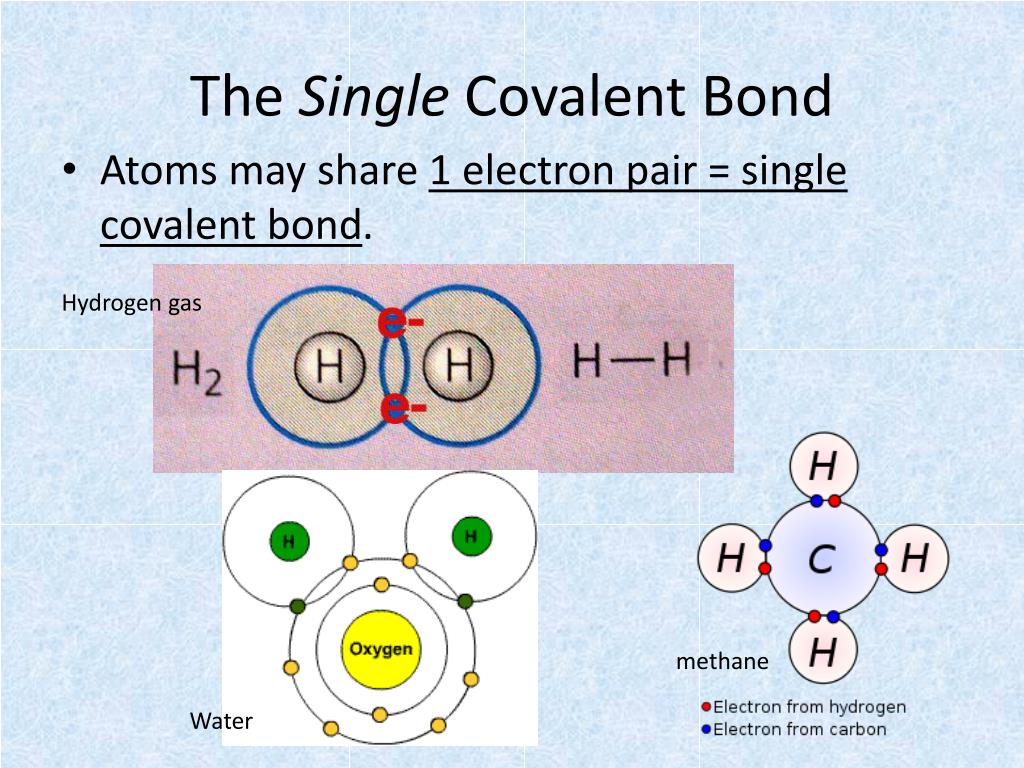
How Many Single Covalent Bonds Does Each Element Generally Form
How Many Single Covalent Bonds Does Each Element Generally Form - Web the number refers to the number of bonds each of the element makes: That is, the atoms share one pair of electrons where the bond. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web how many covalent bonds are formed? Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. Cl + cl cl 2. Molybdenum bromine oxygen potassium nitrogen how many single covalent bonds does each element. Therefore, covalent bonds lead to formation of single, double, triple, and quadruple bonds. Click the card to flip 👆. Single, double, and triple covalent bonds may. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. There is a quick way to work out how many covalent bonds an element will. Molybdenum bromine oxygen potassium nitrogen how many single covalent bonds does each element. Web two pairs of electrons are shared by two atoms to make a double bond.. That is, the atoms share one pair of electrons where the bond. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Web covalent bonds include interactions of the sigma and pi orbitals; Group 5a form 3 bonds; Carbon, for example has four unpaired electrons in its outer energy level. Web covalent bonds involve the sharing of electron pairs between atoms. Click the card to flip 👆. There is a quick way to work out how many covalent bonds an element will. Therefore, it is exothermic.the heat released. Web when it comes to covalent bonding, we only take the valence electrons into account. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web when it comes to covalent bonding, we only take the valence electrons into account. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. Therefore, generally speaking, any atom that doesn't have a full. Single, double, and triple covalent bonds may. Web how many covalent bonds are formed? The potential energy of two separate hydrogen atoms (right) decreases as. Web in the case of cl 2, each atom starts off with seven valence electrons, and each cl shares one electron with the other, forming one covalent bond: Web when it comes to covalent bonding,. Is determined by the distance at which the lowest potential energy is achieved. The potential energy of two separate hydrogen atoms (right) decreases as. Click the card to flip 👆. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Single, double, and triple covalent bonds may. The slideshow shows how a covalent bond forms between a hydrogen atom and a chlorine atom,. Web in chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. Therefore, it is exothermic.the heat released. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach. Carbon, for example has four unpaired electrons in its outer energy level. Web in the case of cl 2, each atom starts off with seven valence electrons, and each cl shares one electron with the other, forming one covalent bond: Web how many covalent bonds can each element form? Web usually each atom contributes one electron to the shared pair. Web how many covalent bonds are formed? Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Is determined by the distance at which the lowest potential energy is achieved. Therefore, it is exothermic.the heat released. Web the number refers to the number of bonds each of the element makes: Single, double, and triple covalent bonds may. Click the card to flip 👆. Web many molecules include single covalent bonds holding atoms together. The slideshow shows how a covalent bond forms between a hydrogen atom and a chlorine atom,. Click the card to flip 👆. The slideshow shows how a covalent bond forms between a hydrogen atom and a chlorine atom,. A single covalent bond is a bond where two atoms share a pair of electrons. Web covalent bonds include interactions of the sigma and pi orbitals; These four elements are widely used when it comes to drawing lewis structures at. Web usually each atom contributes one electron to the shared pair of electrons. Those are the outermost electrons, which may be. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Is determined by the distance at which the lowest potential energy is achieved. Click the card to flip 👆. Web energy is released when the electrons associated with the two hydrogen atoms form a covalent bond. Web covalent bonds involve the sharing of electron pairs between atoms. Single, double, and triple covalent bonds may. Web in chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. Therefore, covalent bonds lead to formation of single, double, triple, and quadruple bonds. Carbon, for example has four unpaired electrons in its outer energy level. Web how many covalent bonds can each element form? Group 6a form 2 bonds; Web which two elements are components of many organic molecules? Web in the case of cl 2, each atom starts off with seven valence electrons, and each cl shares one electron with the other, forming one covalent bond: Click the card to flip 👆.covalent bond Definition, Properties, Examples, & Facts Britannica
PPT Covalent Compounds PowerPoint Presentation, free download ID
The top panel in this figure shows two hydrogen atoms sharing two
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Covalent Bonding The Science and Maths Zone
Covalent Bonding (Biology) — Definition & Role Expii
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Covalent Bond Examples Several Examples of Covalent (molecular) Bonds
Single Covalent Bond Definition and Examples
Related Post: