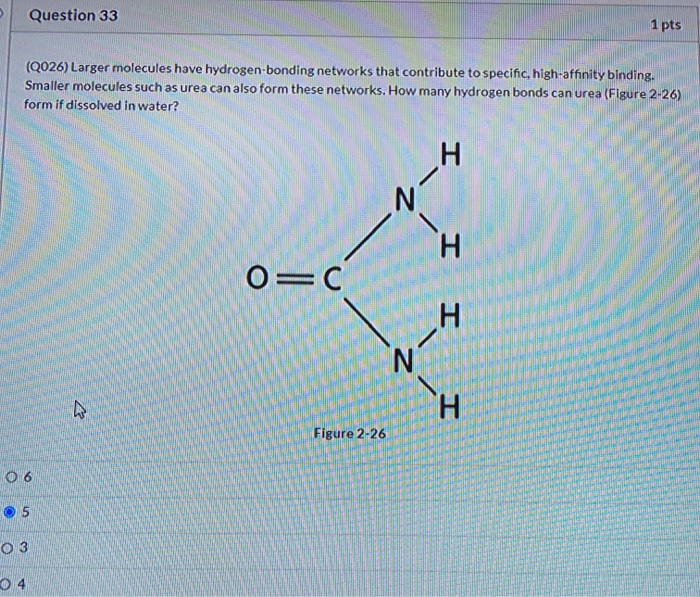
How Many Hydrogen Bonds Can Urea Form
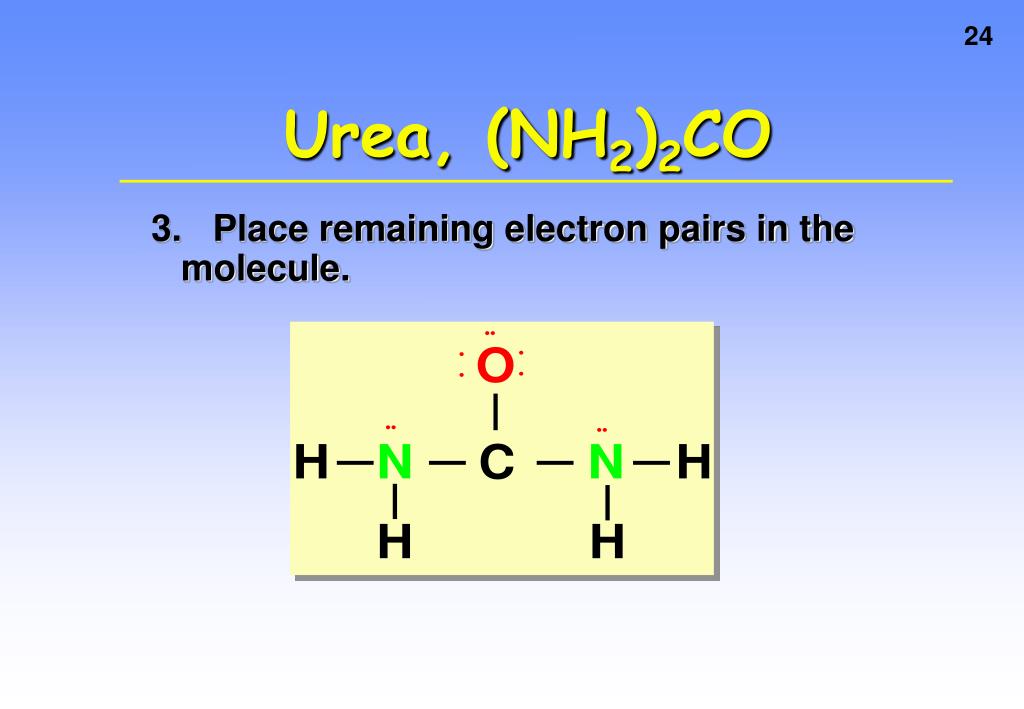
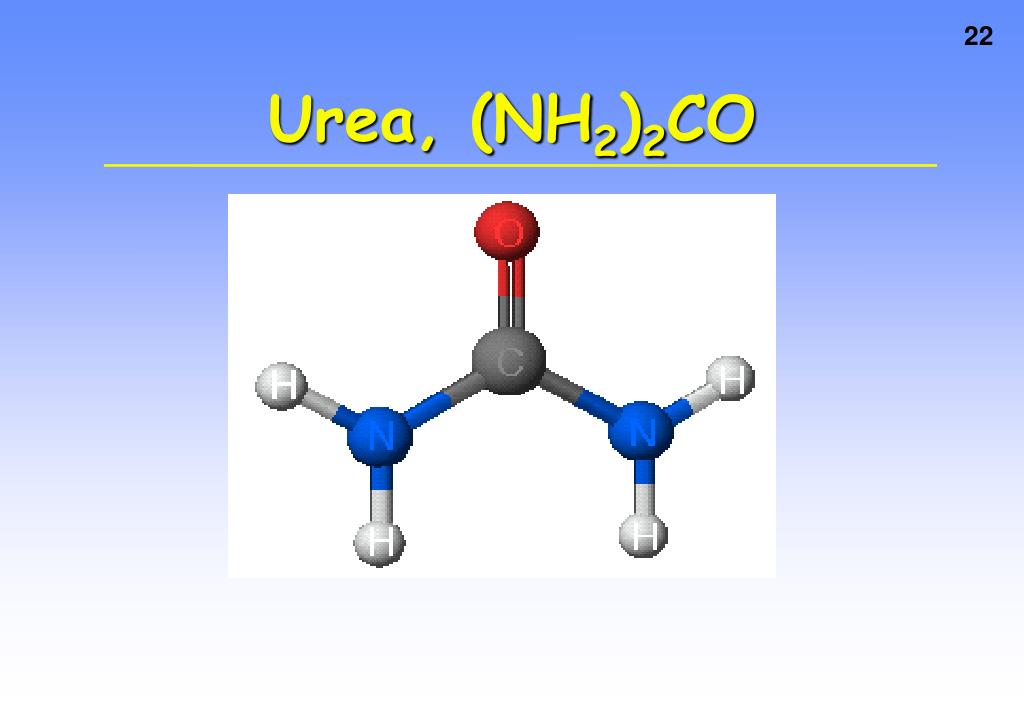
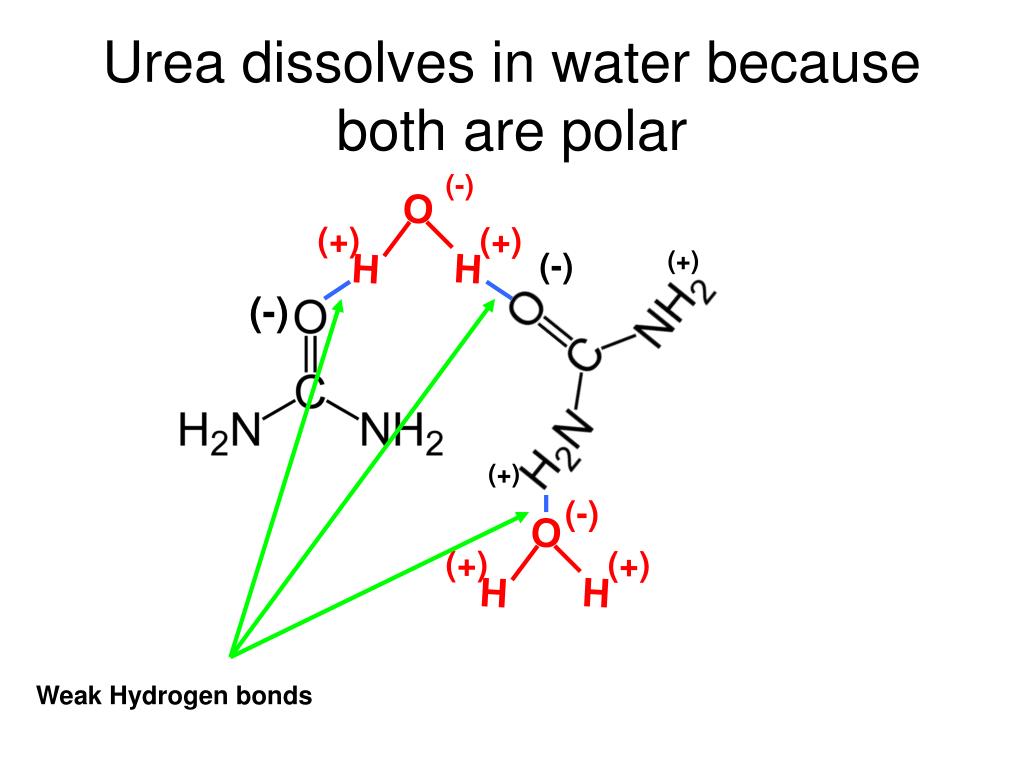
How Many Hydrogen Bonds Can Urea Form - Web this problem has been solved! Does urea have hydrogen bonding? Theoretically, there are a maximum of 5 water molecules that. Web monte carlo simulations [28] show that urea tends to form dimers when the concentration of the solutions is above 6 mol/l and the number of hydrogen bonds. Note, however, that the size and shape of a molecule may limit the number of. One urea molecule can donate a total of four hydrogen bonds to surrounding water molecules. How many hydrogen bond donors does urea have? Web hydrogen bonded chains of urea can be formed in solution due to the large number of sites available on urea molecule for hydrogen bonding. Answer only one, the one at the very top which is attached to the highly electrongative oxygen atom (red), all the others are attached to carbon and can not hydrogen bond. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? O and n atoms can form h bonds (one h bond for each pair of unshared electrons on these atoms; Answer only one, the one at the very top which is attached to the highly electrongative oxygen atom (red), all the others are attached to carbon and can not hydrogen bond. Web as a result, the carbonyl oxygen on each. How many hydrogen bond donors does urea have? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web water molecules forming hydrogen bonds with one another. One urea molecule can donate a total of four hydrogen bonds to surrounding water molecules. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Note, however, that the size and shape of a molecule may limit the number of. Web as a result, the carbonyl oxygen on each urea molecule is hydrogen bonded to four nh groups on three different urea molecules. The urea molecule is planar. H atoms covalently attached to o or n atoms can form h. You'll get a detailed solution. Web how many hydrogen bonds can one urea molecule donate to surrounding water molecules? It has been shown that the linear. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web as a result, the carbonyl oxygen on each urea molecule is hydrogen bonded to four nh groups on three different urea molecules.. O and n atoms can form h bonds (one h bond for each pair of unshared electrons on these atoms; Web jul 22, 2019 · finally, the oxygen in the urea molecule may form hydrogen bonds with water as well, but it has two lone pairs to donate, so the oxygen atom may form. Does urea have hydrogen bonding? Web. The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on. Web as a result, the carbonyl oxygen on each urea molecule is hydrogen bonded to four nh groups on three different urea molecules. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web monte carlo simulations [28]. Web urea could theoretically form hydrogen bonds with this number of water molecules. The urea molecule is planar. Web water molecules forming hydrogen bonds with one another. Does urea have hydrogen bonding? Theoretically, there are a maximum of 5 water molecules that. Web this problem has been solved! Web monte carlo simulations [28] show that urea tends to form dimers when the concentration of the solutions is above 6 mol/l and the number of hydrogen bonds. Web as a result, the carbonyl oxygen on each urea molecule is hydrogen bonded to four nh groups on three different urea molecules. Web how many. The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on. Web as a result, the carbonyl oxygen on each urea molecule is hydrogen bonded to four nh groups on three different urea molecules. Does urea have hydrogen bonding? Web urea could theoretically form hydrogen bonds with this number of. How many hydrogen bond donors does urea have? Web monte carlo simulations [28] show that urea tends to form dimers when the concentration of the solutions is above 6 mol/l and the number of hydrogen bonds. Does urea have hydrogen bonding? Web how many hydrogen bonds can urea form. Web how many hydrogen bonds can one urea molecule donate to. How many hydrogen bond donors does urea have? It has been shown that the linear. Web water molecules forming hydrogen bonds with one another. The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on. Become a study.com member to unlock this answer! Does urea have hydrogen bonding? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web jul 22, 2019 · finally, the oxygen in the urea molecule may form hydrogen bonds with water as well, but it has two lone pairs to donate, so the oxygen atom may form. Theoretically, there are a maximum of 5 water molecules that. Web how many hydrogen bonds can one urea molecule donate to surrounding water molecules? O and n atoms can form h bonds (one h bond for each pair of unshared electrons on these atoms; Web how many hydrogen bonds can urea form. Web monte carlo simulations [28] show that urea tends to form dimers when the concentration of the solutions is above 6 mol/l and the number of hydrogen bonds. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? One urea molecule can donate a total of four hydrogen bonds to surrounding water molecules. H atoms covalently attached to o or n atoms can form h. Note, however, that the size and shape of a molecule may limit the number of. Web hydrogen bonded chains of urea can be formed in solution due to the large number of sites available on urea molecule for hydrogen bonding. The urea molecule is planar. Web as a result, the carbonyl oxygen on each urea molecule is hydrogen bonded to four nh groups on three different urea molecules.PPT CHEMICAL BONDING PowerPoint Presentation, free download ID323318
PPT Chapter 22 Properties of Water PowerPoint Presentation, free
Question 5f606 Socratic
How Many Hydrogen Bonds Can Urea Form
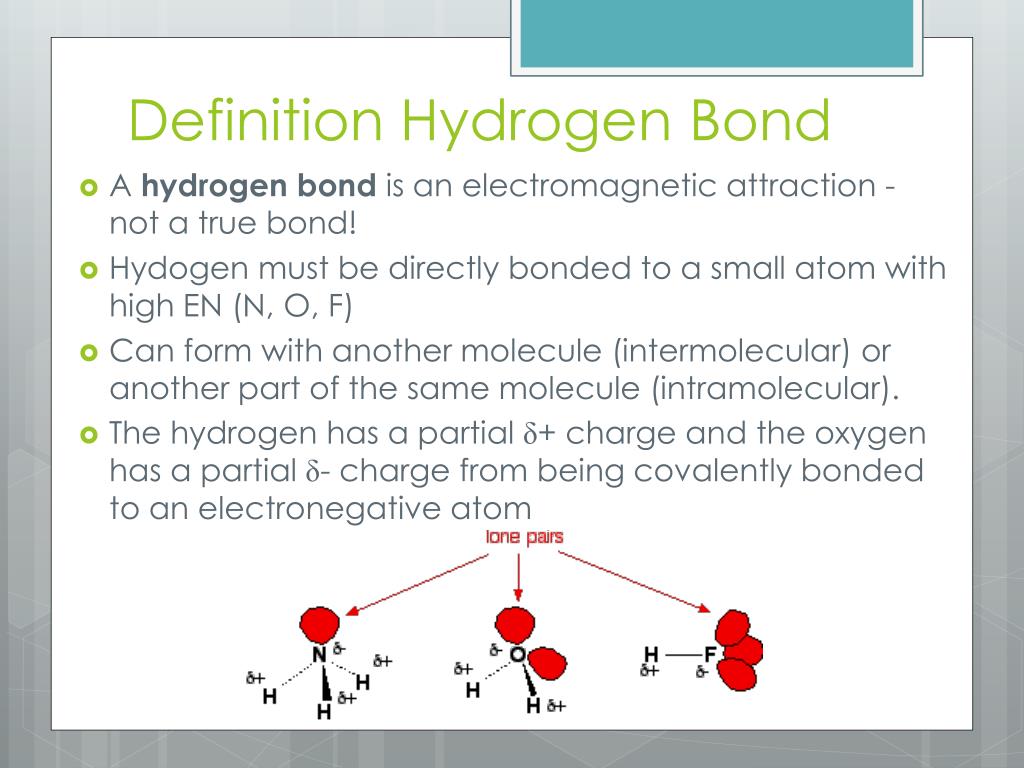
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Effect of urea on the structural dynamics of water PNAS
PPT CHEMICAL BONDING PowerPoint Presentation, free download ID323318
The Many Uses Of Urea
Postulated hydrogen bonds formed between urea, ChCl and water, (a
Figure 1 from Urea frameworks as effective and size
Related Post:
.png)