How Many Covalent Bonds Does Phosphorus Form
How Many Covalent Bonds Does Phosphorus Form - Web covalent radius half of the distance between two atoms within a single covalent bond. Similarly, the phosphorus atom in \(\ce{pcl_5}\) has 10 valence. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its. Web typically, the atoms of group 4a form 4 covalent bonds; Atoms with 5 valence electrons typically. Web covalent bonds involve the sharing of electron pairs between atoms. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. Web how does phosphorus form 5 covalent bonds? And group 7a form one bond. Web meallic elements can definiely have more than eight valence electrons, however they do not tend to form covalent bonds. Values are given for typical oxidation number and coordination. Web typically, the atoms of group 4a form 4 covalent bonds; Group 5a form 3 bonds; Group 6a form 2 bonds; Group 5a form 3 bonds; Group 6a form 2 bonds; Web how does phosphorus form 5 covalent bonds? Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Values are given for typical oxidation number and coordination. Web covalent radius half of the distance between two atoms within a single covalent bond. Web covalent bonds involve the sharing of electron pairs between atoms. Web covalent radius half of the distance between two atoms within a single covalent bond. Group 6a form 2 bonds; In each case, the sum. Web how does phosphorus form 5 covalent bonds? In each case, the sum. The potential energy of two separate hydrogen atoms (right) decreases as. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Web typically, the atoms of group 4a form 4 covalent bonds; Therefore the maximum number of covalent bonds. Similarly, the phosphorus atom in \(\ce{pcl_5}\) has 10 valence. How many bonds does phosphorus typically make? Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. How many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom? Electron pairs shared. Web there are many different modifications of phosphorus in nature. Web how does phosphorus form 5 covalent bonds? Covalent bond two atoms form a covalent chemical. Web covalent bonds involve the sharing of electron pairs between atoms. This is summarized in the table below. Web covalent bonds involve the sharing of electron pairs between atoms. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web there are many different modifications of phosphorus in nature. Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds. Web typically,. Web select all statements that correctly describe the typical number of covalent bonds formed by common neutral atoms. Web typically, the atoms of group 4a form 4 covalent bonds; This is summarized in the table below. Therefore the maximum number of covalent bonds. Web there are many different modifications of phosphorus in nature. Group 5a form 3 bonds; Web typically, the atoms of group 4a form 4 covalent bonds; Web typically, the atoms of group 4a form 4 covalent bonds; In each case, the sum. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web meallic elements can definiely have more than eight valence electrons, however they do not tend to form covalent bonds. Web how does phosphorus form 5 covalent bonds? Is determined by the distance at which the lowest potential energy is achieved. How many bonds does phosphorus typically make? And group 7a form one bond. Is determined by the distance at which the lowest potential energy is achieved. Covalent bond two atoms form a covalent chemical. How many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom? Therefore the maximum number of covalent bonds. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its. This is summarized in the table below. Group 5a form 3 bonds; Web covalent radius half of the distance between two atoms within a single covalent bond. Group 6a form 2 bonds; Web covalent bonds involve the sharing of electron pairs between atoms. Web typically, the atoms of group 4a form 4 covalent bonds; In each case, the sum. How many valence electrons are present in the atom al? Web typically, the atoms of group 4a form 4 covalent bonds; Similarly, the phosphorus atom in \(\ce{pcl_5}\) has 10 valence. Group 6a form 2 bonds; Web there are many different modifications of phosphorus in nature. Web how many bonds does phosphorus typically make? Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
41. What is the Lewis dot structure of phosphate ion? how many
Periodic Table Phosphorus Valence Electrons Periodic Table Timeline
How Many Valence Electrons Are Found In Phosphorus
What type of bond would form between two atoms of phosphorus? A. Triple
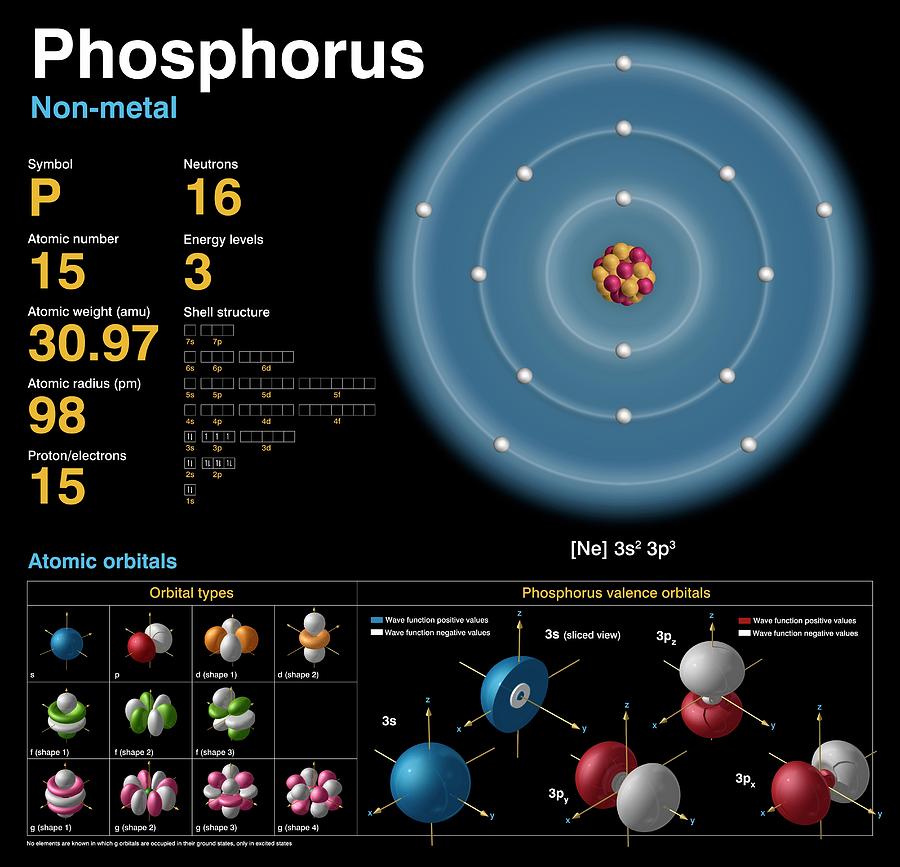
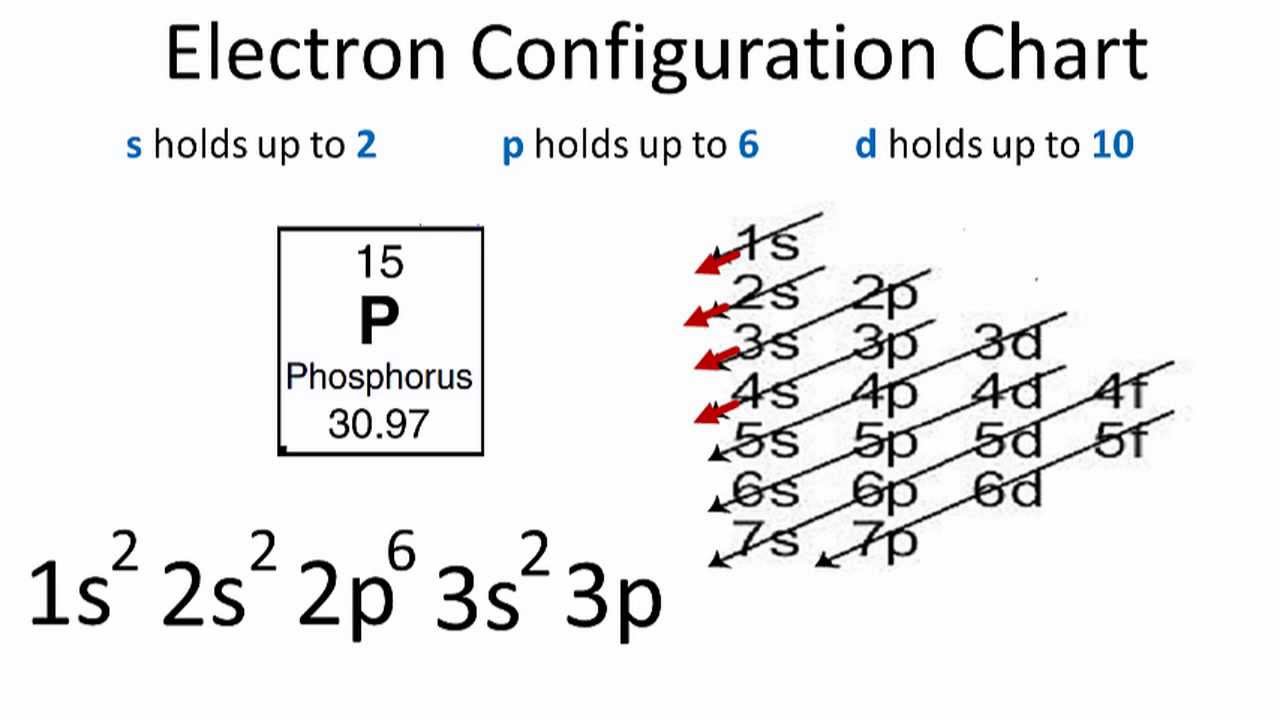
Phosphorus Electron Configuration (P) with Orbital Diagram
What Is Covalent Bonding?
Chapter 8 Covalent Bonding Covalent bonding Usually forms
How does phosphorus form 5 covalent bonds? The Unconditional Guru
covalent bond Definition, Properties, Examples, & Facts Britannica
Related Post: