How Many Covalent Bonds Can Hydrogen Form
How Many Covalent Bonds Can Hydrogen Form - Web how many covalent bonds can hydrogen form? Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3,. Web and now here, once again, oxygen can kinda pretend like it has eight valence electrons, two, four, six, eight. Web a covalent bond is formed between two atoms by sharing electrons. Nitrogen atoms are composed of electrons, protons, and neutrons. This is because the oxygen atom, in addition to forming bonds. Web biology biology questions and answers a hydrogen atom has one electron. Web how many covalent bonds can hydrogen form?, a hypothesis, a solution with a ph of 7 is and more. Have more neutrons than the usual nitrogen atom. For example, the hydrogen molecule, h 2, contains a covalent bond. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web how many covalent bonds can hydrogen form? Web this comes in handy especially when drawing lewis structures. Web when a covalent bond is formed, the atomic orbitals (the orbitals in the individual atoms) merge to produce a new molecular. Covalent and ionic bonds are both typically considered strong bonds. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three. Web this comes in handy especially when drawing lewis structures. This is because the oxygen atom, in addition to forming bonds. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two hydrogen atoms. The number refers to the number of bonds each. Hydrogen is an example of an extremely. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. However, other kinds of more temporary bonds can. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. This is because the oxygen atom, in addition to forming bonds. Web hydrogen bonds and london dispersion forces. Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3,. For example,. Hydrogen is an example of an extremely. Study with quizlet and memorize flashcards containing terms like a hydrogen. Web each water molecule can form up to four hydrogen bonds, because they have two partially positively charged hydrogen atoms and two electron lone pairs. This is because the oxygen atom, in addition to forming bonds. Web hydrogen bonds and london dispersion. Web how many covalent bonds can hydrogen form?, a hypothesis, a solution with a ph of 7 is and more. Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond. Covalent and ionic bonds are both typically considered strong bonds. Web a covalent bond. Web formation of covalent bonds. Have more neutrons than the usual nitrogen atom. Web a covalent bond is a chemical bond that comes from the sharing of one or more electron pairs between two atoms. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Nonmetal atoms frequently form covalent. It’s called the honc rule, or sometimes known as honc 1234 rule. Hydrogen is an example of an extremely. Study with quizlet and memorize flashcards containing terms like a hydrogen. Web and now here, once again, oxygen can kinda pretend like it has eight valence electrons, two, four, six, eight. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a. Web biology biology questions and answers a hydrogen atom has one electron. Web hydrogen bonds and london dispersion forces. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three. Hydrogen is an example of an extremely. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web and now here, once again, oxygen can kinda pretend like it has eight valence electrons, two, four, six, eight. However, other kinds of more temporary bonds can. Web although. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web each water molecule can form up to four hydrogen bonds, because they have two partially positively charged hydrogen atoms and two electron lone pairs. H forms only one bond because it needs only two electrons. Web formation of covalent bonds. Web and now here, once again, oxygen can kinda pretend like it has eight valence electrons, two, four, six, eight. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two hydrogen atoms. For example, the hydrogen molecule, [latex]\ce{h2}[/latex], contains a. Web when a covalent bond is formed, the atomic orbitals (the orbitals in the individual atoms) merge to produce a new molecular orbital which contains the electron. It’s called the honc rule, or sometimes known as honc 1234 rule. Web how many covalent bonds can hydrogen form? Web two different atoms can also share electrons and form covalent bonds. Study with quizlet and memorize flashcards containing terms like a hydrogen. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three. The number of bonds an element forms in a covalent compound is determined by the number of electrons it. Covalent and ionic bonds are both typically considered strong bonds. Web a covalent bond is a chemical bond that comes from the sharing of one or more electron pairs between two atoms. This is because the oxygen atom, in addition to forming bonds. Web hydrogen bonds and london dispersion forces. Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3,. The number refers to the number of bonds each.PPT Covalent bonding in hydrogen PowerPoint Presentation, free
Hydrogen Bonds — Overview & Examples Expii
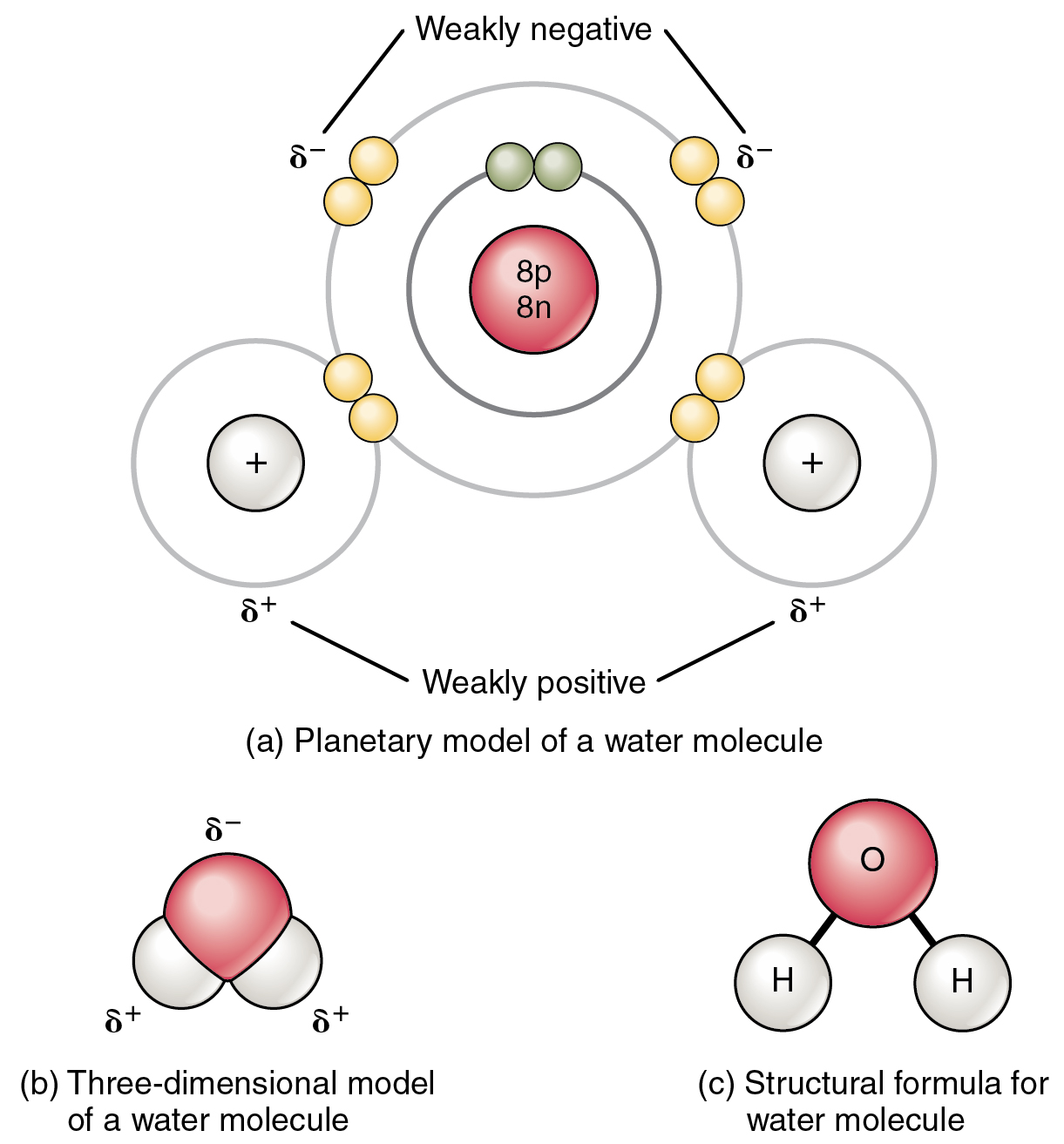
Chemical Bonds · Anatomy and Physiology
How many covalent bonds can Hydrogen form? YouTube
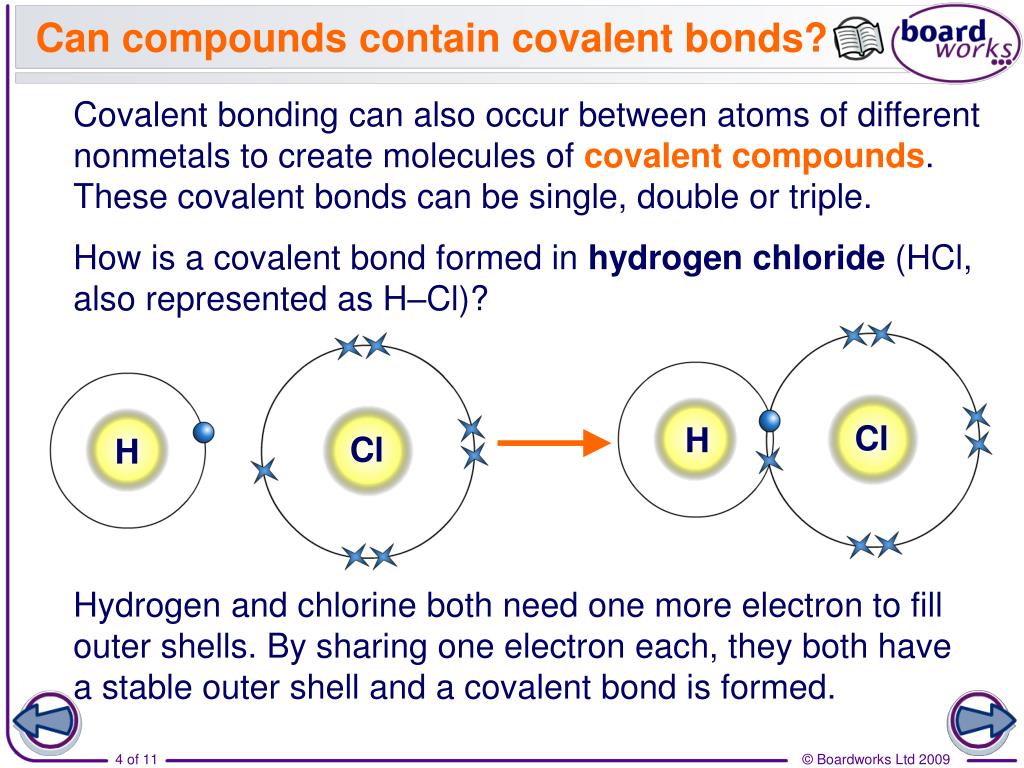
PPT Covalent bonding in hydrogen PowerPoint Presentation, free
covalent bond Definition, Properties, Examples, & Facts Britannica
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
The top panel in this figure shows two hydrogen atoms sharing two
PPT Covalent bonding in hydrogen PowerPoint Presentation, free
Question Video Determining the Number of Covalent Bonds That Hydrogen
Related Post: