How Many Covalent Bonds Can Chlorine Form
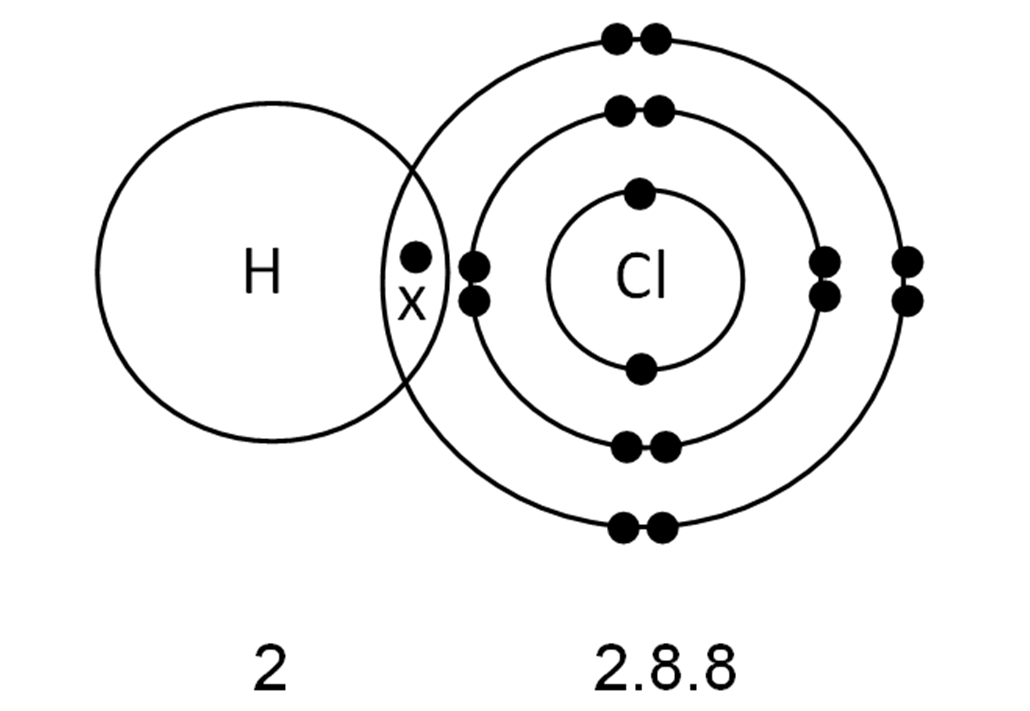
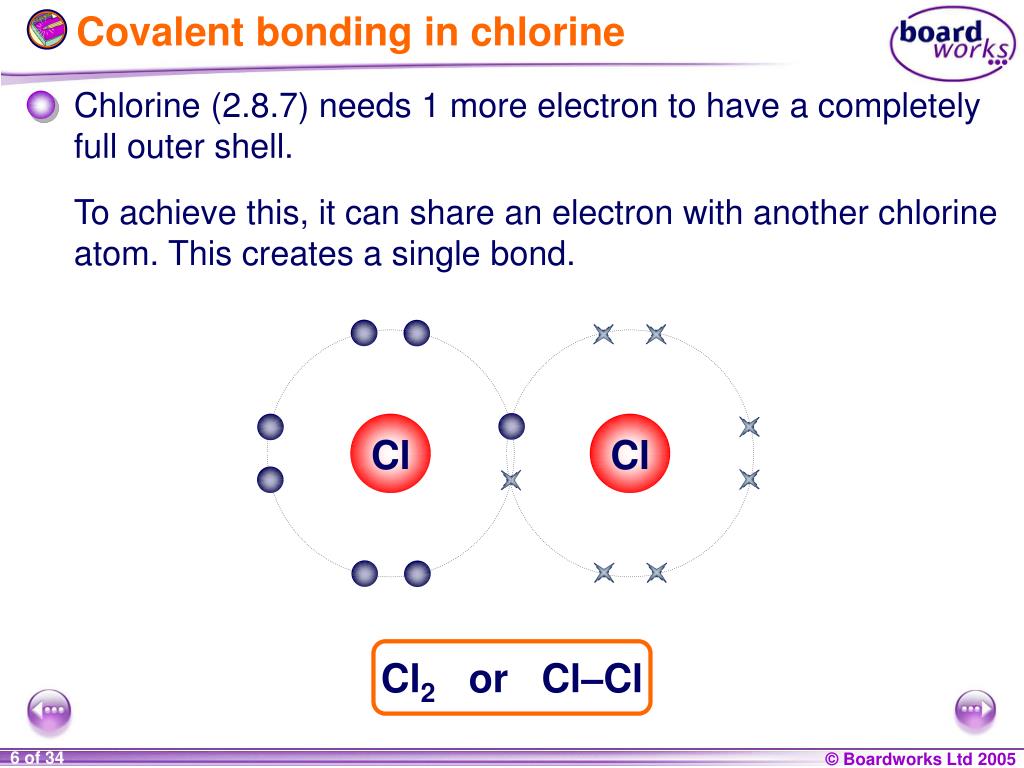
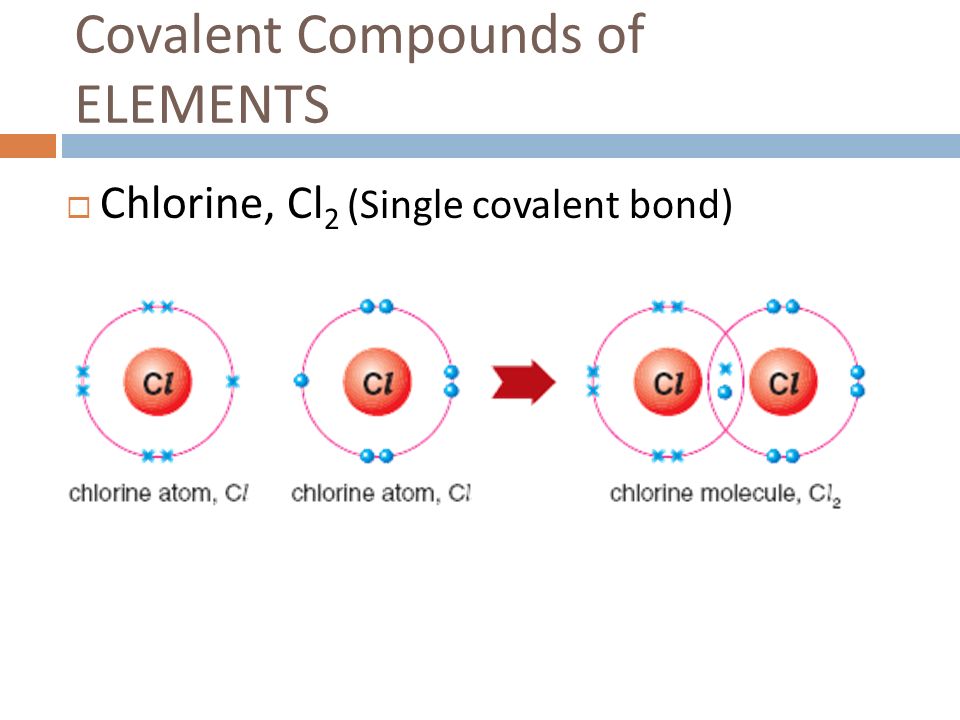
How Many Covalent Bonds Can Chlorine Form - In molecules, there is a pattern to the number of covalent bonds that different atoms can form. Web it forms strong ionic bonds with metal ions. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web generally, two models exist. Web a hydrogen atom with one electron and a chlorine atom with 17 electrons. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. Web how many covalent bonds can chlorine form? Web the hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. Is determined by the distance at which the lowest potential energy is achieved. Web there are 3 unpaired electrons that can be used to form bonds with 3 chlorine atoms. Web when participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. Web how many covalent bonds can chlorine form? Web diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. Another way the octet rule can be satisfied is by the sharing of electrons between atoms to form covalent bonds. In molecules, there. How do ionic bonds differ from covalent bonds in terms of how electrons are shared ? Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\). Web how many covalent bonds can chlorine form? Web chlorine (cl), on the other hand, has seven electrons in its outer shell. Web when participating in. Web how many covalent bonds are formed? Web a hydrogen atom with one electron and a chlorine atom with 17 electrons. Another way the octet rule can be satisfied is by the sharing of electrons between atoms to form covalent bonds. However, the carbon, oxygen, and nitrogen atoms can bond. Is determined by the distance at which the lowest potential. Why do nonmetals near the. Is determined by the distance at which the lowest potential energy is achieved. Web when participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence.. This is summarized in the table below. Web diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. How do ionic bonds differ from covalent bonds in terms of how electrons are shared ? Web how many covalent bonds can chlorine form? Web when participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. Web how many covalent bonds can chlorine form? The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence. Web it forms strong ionic bonds with metal ions. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\).. Web generally, two models exist. Web meallic elements can definiely have more than eight valence electrons, however they do not tend to form covalent bonds. Web how many covalent bonds can chlorine form? Web how many covalent bonds are formed? It is possible to move some electrons. Web how many covalent bonds can chlorine form? Web how many covalent bonds can chlorine form? However, the carbon, oxygen, and nitrogen atoms can bond. Web generally, two models exist. Web there are 3 unpaired electrons that can be used to form bonds with 3 chlorine atoms. Web the hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. Web diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. How do ionic bonds differ from covalent bonds in terms of how electrons are shared ? As it has one electron to start with, it can only make. Therefore the maximum number of covalent bonds should. In molecules, there is a pattern to the number of covalent bonds that different atoms can form. Web that also means, it can form multiple number of bonds. How do ionic bonds differ from covalent bonds in terms of how electrons are shared ? How many covalent bonds are formed? Containing covalent bonds between two of the same type of atom are only a few examples of the vast number of molecules that can form. Web that also means, it can form multiple number of bonds. Why do nonmetals near the. Web when participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. How do ionic bonds differ from covalent bonds in terms of how electrons are shared ? If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. Web there are 3 unpaired electrons that can be used to form bonds with 3 chlorine atoms. In this case, it is easier for chlorine to gain one electron than to lose seven, so it tends to take on an. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet. Web meallic elements can definiely have more than eight valence electrons, however they do not tend to form covalent bonds. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\). Web the hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. Another way the octet rule can be satisfied is by the sharing of electrons between atoms to form covalent bonds. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence. Web a hydrogen atom with one electron and a chlorine atom with 17 electrons. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. It is possible to move some electrons. Web how many covalent bonds can chlorine form? The potential energy of two separate hydrogen atoms (right) decreases as. In molecules, there is a pattern to the number of covalent bonds that different atoms can form.__TOP__ How Many Covalent Bonds Can Chlorine Form
Chem Easy Formation of covalent bond in chlorine molecule
Introducing Covalent Bonding Montessori Muddle
Bond Formation In Chlorine Molecule Photograph by
Expanded Electron Configuration of Chlorine Womack Thille
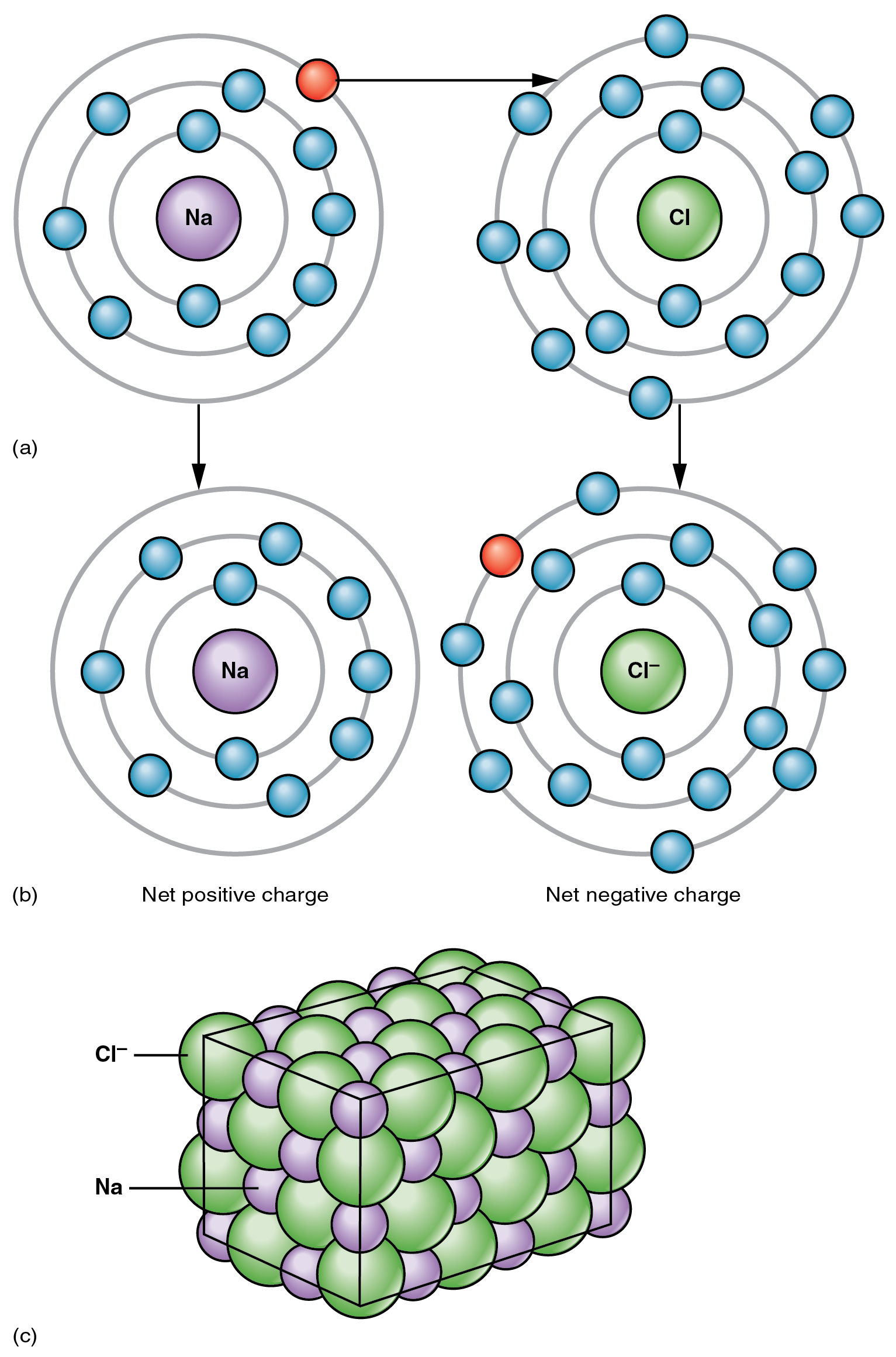
The top panel of this figure shows the orbit model of a sodium atom and
A chlorine molecule forms a covalent bond Electronics And Engineering Lab
PPT KS4 Chemistry PowerPoint Presentation, free download ID2755080
Diagram Of A Chlorine Atom Photos
covalent bonding of chlorine Brainly.in
Related Post: