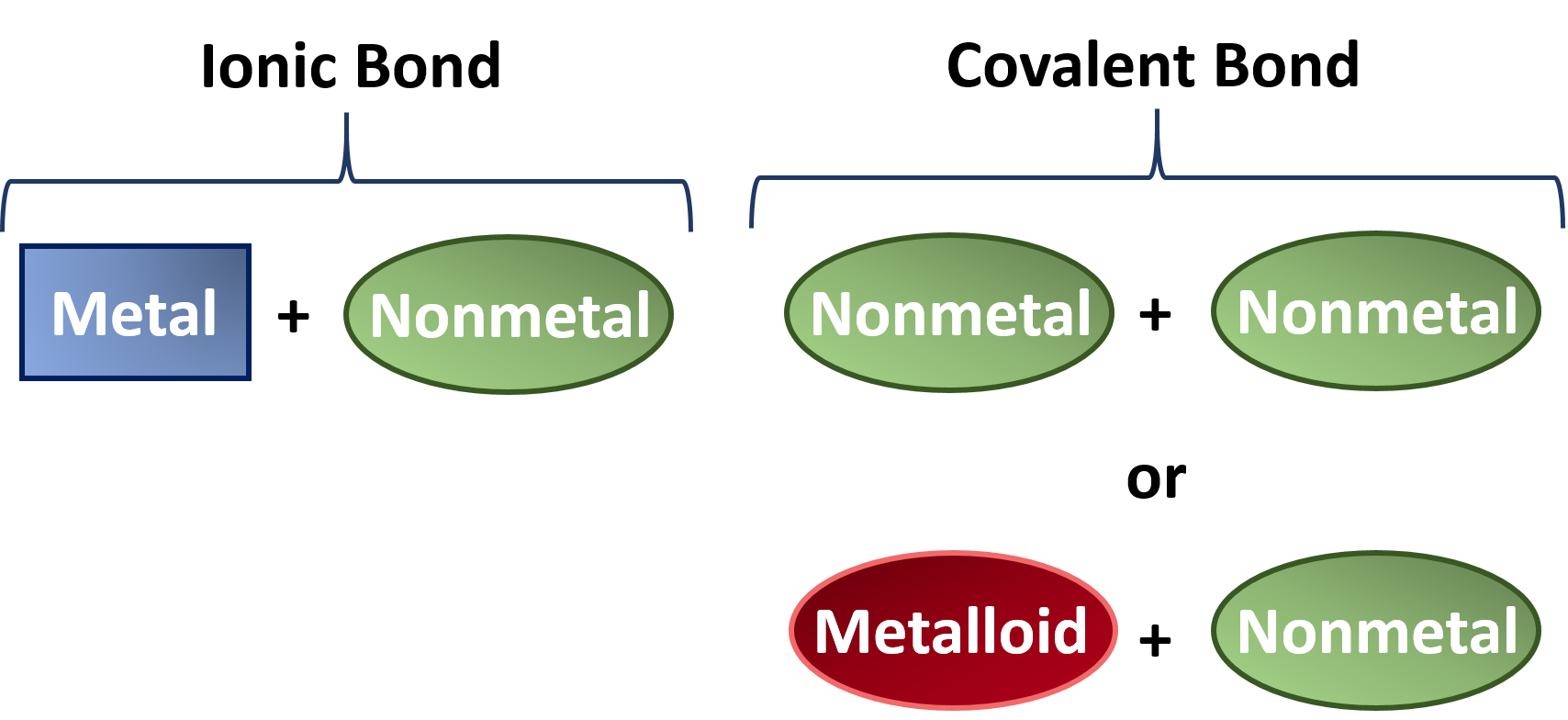
How Many Covalent Bonds Can Boron Form
How Many Covalent Bonds Can Boron Form - The size of b x 3 + ions, is very small and the ion has high charge density. Also because of its size the sum of its first three ionization enthalpies is very high. Carbon (represented as c) is in group 4a and has 4 valence. Therefore, the number of covalent bonds boron can form by sharing its. There is a quick way to work out how many covalent bonds an element will form. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Thus, following fajan's rule, boron forms covalent compounds. Web so, the number of single covalent bonds formed by boron is three. This is summarized in the table below. In the case of boron in bf 3, three bonds is the maximum possible because. The potential energy of two separate hydrogen atoms (right) decreases as. Web the most common examples are the covalent compounds of beryllium and boron. Web covalent radius half of the distance between two atoms within a single covalent bond. Web it follows, therefore, that an atom will tend to make as many covalent bonds as possible. Boron forms covalent bonds. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web that also means, it can form multiple number of bonds. Web while most atoms obey the duet and octet rules, there are some exceptions. Web for example, boron (represented as b in the periodic table) is in group 3a. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Covalent compounds contain covalent bonds. Values are given for typical oxidation number and coordination. In the case of boron in bf 3, three bonds is the maximum possible because boron only has. There is a. Nitrogen, n2, is a covalent compound. Typically, boron forms 3 covalent bonds. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. The potential energy of two separate hydrogen atoms (right) decreases as. We know that there are two modes of chemical combination, that is, by. Boron forms covalent bonds due to small size of boron. Web the valency of an atom is the maximum number of bonds it can form, usually the number of electrons required to reach a stable noble gas configuration (there are. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four). Web the most common examples are the covalent compounds of beryllium and boron. Boron can form a fourth covalent bond and thus acquire a formal negative charge. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Web it follows, therefore, that an atom will. Covalent compounds contain covalent bonds. Web option d is a correct answer. This is summarized in the table below. Boron can form a fourth covalent bond and thus acquire a formal negative charge. Therefore, the number of covalent bonds boron can form by sharing its. In the case of boron in bf 3, three bonds is the maximum possible because. In the case of boron in bf 3, three bonds is the maximum possible because boron only has. Web so, the number of single covalent bonds formed by boron is three. Thus, following fajan's rule, boron forms covalent compounds. Web while most atoms obey the. Nitrogen, n2, is a covalent compound. Therefore, the number of covalent bonds boron can form by sharing its. Web option d is a correct answer. In each case, the sum. Web since boron only has three valence electrons, it can only form three covalent bonds maximum. Web option d is a correct answer. Typically, boron forms 3 covalent bonds. For example, elements such as boron or beryllium often form compounds in which the central atom is. Web the valency of an atom is the maximum number of bonds it can form, usually the number of electrons required to reach a stable noble gas configuration (there are.. In the case of boron in bf 3, three bonds is the maximum possible because boron only has. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Covalent compounds contain covalent bonds. Carbon (represented as c) is in group 4a and has 4 valence. This is summarized in the table below. Web it follows, therefore, that an atom will tend to make as many covalent bonds as possible. Boron can form a fourth covalent bond and thus acquire a formal negative charge. Web the valency of an atom is the maximum number of bonds it can form, usually the number of electrons required to reach a stable noble gas configuration (there are. Therefore, the number of covalent bonds boron can form by sharing its. Web since boron only has three valence electrons, it can only form three covalent bonds maximum. Web option d is a correct answer. Is determined by the distance at which the lowest potential energy is achieved. Also because of its size the sum of its first three ionization enthalpies is very high. Nitrogen, n2, is a covalent compound. In the case of boron in bf 3, three bonds is the maximum possible because. In each case, the sum. Web boron has a relatively limited tendency to form complexes, but aluminum, gallium, indium, and, to some extent, thallium form many complexes. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web so, the number of single covalent bonds formed by boron is three.covalent bond Definition, Properties, Examples, & Facts Britannica
SiliconBoron Covalent Bond ECHEMI
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
Boron Boundless Chemistry
Unprecedented formation of a boronboron covalent bond opens a new
Reading Covalent Bonds Biology I
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Boron Properties, Uses, & Facts Britannica
crystal Types of bonds Britannica
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Related Post: