How Many Bonds Can Oxygen Form
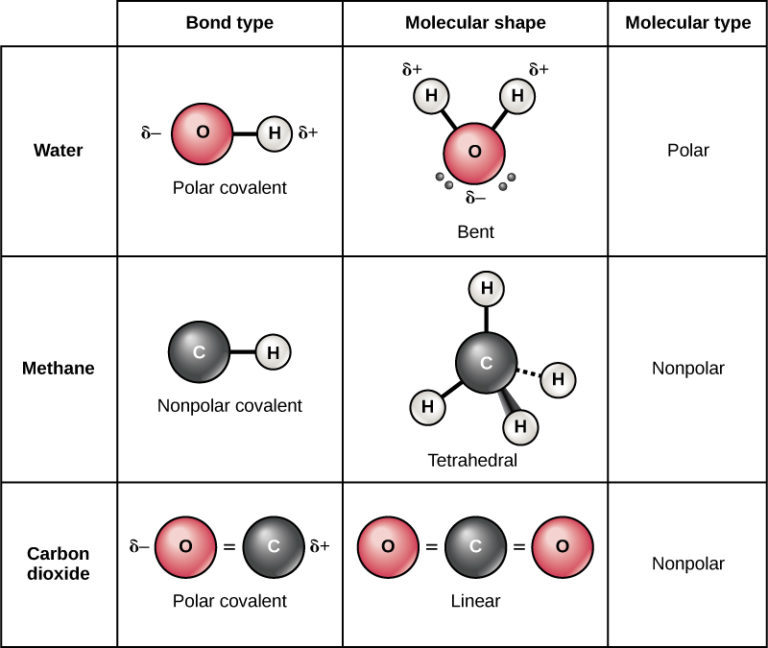
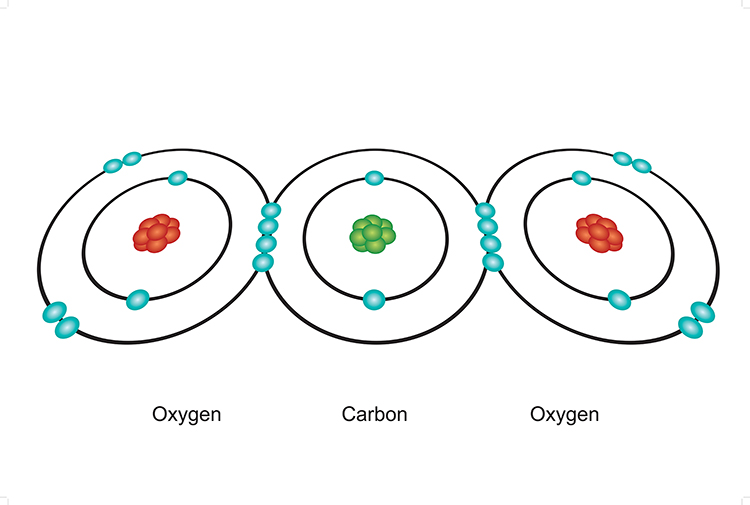
How Many Bonds Can Oxygen Form - They become a cl 2 molecule. It tends to form either two single covalent bonds or one. The first oxygen has three bonds, the second only has one. Web compounds containing oxygen in other oxidation states are very uncommon: Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web we would like to show you a description here but the site won’t allow us. Web oxygen and other atoms in group 6a obtain an octet by forming two covalent bonds. Web two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). You can think of the reaction taking place by a lone pair on the oxygen of one water. Web 1 how many bonds does oxygen typically form? Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Fluorine and the other halogens in group 7a have seven valence electrons and can. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Does this make sense based. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web we would like to show you a description here but the site won’t allow us. Web how many single covalent bonds can oxygen form? They become a cl 2 molecule. Two core and seven valence. Organic chemistry lewis structures and bonding covalent bonds 1 answer scooke feb 27, 2016 two,. By sharing two pairs of valence electrons, each oxygen atom has a total of eight valence electrons. − 1 ⁄ 2 (superoxides), − 1 ⁄ 3 , 0 (elemental, hypofluorous acid), + 1 ⁄ 2 , +1 (dioxygen difluoride),. Web to obtain an octet, these. It tends to form either two single covalent bonds or one. Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3,. Two core and seven valence. − 1 ⁄ 2 (superoxides), − 1 ⁄ 3 , 0 (elemental, hypofluorous acid),. Nitrogen atoms will form three covalent bonds. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). It tends to form either two single covalent bonds or one. You can think of the reaction taking place by a lone. By sharing two pairs of valence electrons, each oxygen atom has a total of eight valence electrons. Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3,. Oxygen and other atoms in group 16 obtain an octet by forming two. Web compounds containing oxygen in other oxidation states are very uncommon: Oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3,. Organic chemistry lewis. Web two chlorine atoms can share 1 electron each to form a single covalent bond. Oxygen and other atoms in group 6a (16) obtain an octet by forming two. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web oxygen and other atoms in group 6a (16) obtain an octet by forming two. Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3,. Web two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. Web alone, each oxygen atom of group 6a or. Two core and seven valence. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: Web two chlorine atoms can share 1 electron each to form a single covalent bond. − 1 ⁄ 2 (superoxides), − 1 ⁄ 3 , 0 (elemental, hypofluorous acid), + 1 ⁄ 2 , +1 (dioxygen difluoride),. Web to obtain. Web 1 how many bonds does oxygen typically form? Web oxygen is a group 16 element, and it can gain the same electron configuration as neon if it makes two covalent bonds. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: Does this make sense based on the number of valence electrons in an oxygen atom? You can think of the reaction taking place by a lone pair on the oxygen of one water. Web two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Nitrogen atoms will form three covalent bonds. Web two chlorine atoms can share 1 electron each to form a single covalent bond. By sharing two pairs of valence electrons, each oxygen atom has a total of eight valence electrons. Web we would like to show you a description here but the site won’t allow us. Oxygen and other atoms in group 6a (16) obtain an octet by forming two. Web alone, each oxygen atom of group 6a or group 16 has six valence electrons. Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3,. Fluorine and the other halogens in group 7a have seven valence electrons and can. Web compounds containing oxygen in other oxidation states are very uncommon: Organic chemistry lewis structures and bonding covalent bonds 1 answer scooke feb 27, 2016 two,. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). − 1 ⁄ 2 (superoxides), − 1 ⁄ 3 , 0 (elemental, hypofluorous acid), + 1 ⁄ 2 , +1 (dioxygen difluoride),.Covalent bonding in an oxygen molecule. Chemistry Activities, Gcse
Researchers discover a way to tease oxygen molecules from carbon dioxide
Covalent Bonds Biology for NonMajors I
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Question 4e4a3 Socratic
Why does oxygen only forms only 2 hydrogen bonds
The Covalent Bond CK12 Foundation
Chemical Bonds Anatomy and Physiology I
Online Essay Help amazonia.fiocruz.br
Covalent bonding is where atoms share electrons by bonding
Related Post: