How Many Bonds Can Beryllium Form
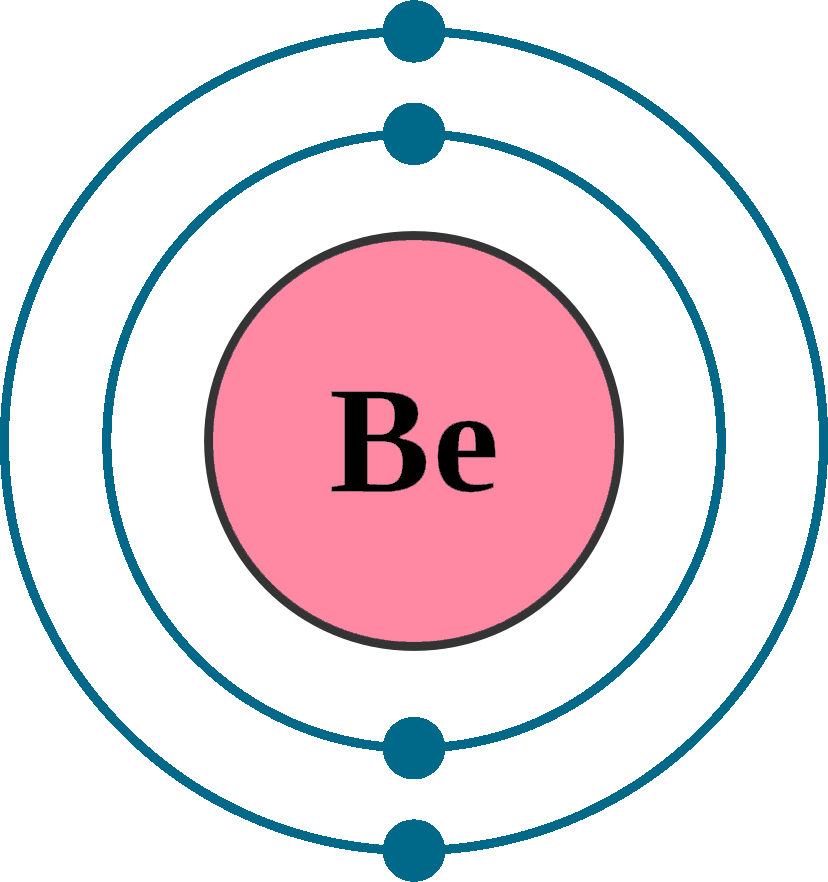
How Many Bonds Can Beryllium Form - Beryllium is used industrially in three forms: As the compound transitions from the gas phase to the solid phase, many beryllium chloride molecules combine to form the extended linear structure. Incompatibilities & reactivities acids, caustics, chlorinated. Statistics and information on the worldwide supply of, demand for, and flow of the mineral commodity beryllium. Web bertrandite (<1% beryllium) is the principal mineral mined for beryllium in the u.s. Web beryllium ores and concentrates 2617.90.0030 free. Web we can see that the molecule has two covalent bonds but no coordinate covalent bonds, as each shared pair of electrons has one from each bonding atom. In addition, eight criteria based on qtaim properties, including the values of electron density and its laplacian at the bcp, penetration of beryllium and acceptor atom, charge. Web the description of the element in its natural form. Noncombustible solid in bulk form, but a slight explosion hazard in the form of a powder or dust. Thickness of 5 millimeters (mm) or more 7409.90.1030 3.0% ad val. Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. Web the molecular graphs of the investigated complexes indicate that beryllium in beh2 and bef2 can form “beryllium bonds” with o, n and p atoms but not with halogens. 14, tetrel bonds. Beryllium forms an oxide layer making it not react with air or water even in extreme heat. Web the molecular graphs of the investigated complexes indicate that beryllium in beh2 and bef2 can form “beryllium bonds” with o, n and p atoms but not with halogens. Values are given for typical. Boron commonly makes only three covalent bonds, resulting in. Statistics and information on the worldwide supply of, demand for, and flow of the mineral commodity beryllium. Web the molecular graphs of the investigated complexes indicate that beryllium in beh2 and bef2 can form “beryllium bonds” with o, n and p atoms but not with halogens. Web beryllium forms two single covalent bonds in becl2 with chlorine atoms. In a. As a pure metal, as beryllium oxide, and most commonly, as an alloy with copper, aluminum, magnesium, or nickel. While beryl (4% beryllium) is the principal mineral mined for beryllium in the rest of the world. In addition, eight criteria based on qtaim properties, including the values of electron density and its laplacian at the bcp, penetration of beryllium and. Covalent bonds are formed when two atoms share electrons. Thickness of 5 millimeters (mm) or more 7409.90.1030 3.0% ad val. Web bertrandite (<1% beryllium) is the principal mineral mined for beryllium in the u.s. While beryl (4% beryllium) is the principal mineral mined for beryllium in the rest of the world. Web we can see that the molecule has two. Half of the distance between two atoms within a single covalent bond. Since beryllium is an alkaline earth metal, the bonds between beryllium atoms could be considered metallic and we can use molecular orbital theory (mot) to explain metallic bonds in metals. Beryllium oxide and hydroxide 2825.90.1000 3.7% ad val. Ds1750000 (metal) dot id & guide. Web for example, beryllium. Beryllium is used industrially in three forms: The role of the element in humans, animals and plants. Beryllium (be) forms single covalent bonds in becl2. Web bertrandite (<1% beryllium) is the principal mineral mined for beryllium in the u.s. As the compound transitions from the gas phase to the solid phase, many beryllium chloride molecules combine to form the extended. Web the molecular graphs of the investigated complexes indicate that beryllium in beh2 and bef2 can form “beryllium bonds” with o, n and p atoms but not with halogens. Incompatibilities & reactivities acids, caustics, chlorinated. 14, tetrel bonds (old group iv) 159; If beryllium dust or fumes are inhaled, it can lead to an incurable inflammation of the lungs called. Thickness of 5 millimeters (mm) or more 7409.90.1030 3.0% ad val. In addition, eight criteria based on qtaim properties, including the values of electron density and its laplacian at the bcp, penetration of beryllium and acceptor atom, charge. Web we can see that the molecule has two covalent bonds but no coordinate covalent bonds, as each shared pair of electrons. Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. In addition, eight criteria based on qtaim properties, including the values of electron density and its laplacian at the bcp, penetration of beryllium and acceptor atom, charge. Thickness of less than 5 mm: Web for example, beryllium can form two covalent bonds, resulting. Beryllium is used industrially in three forms: Beryllium (be) forms single covalent bonds in becl2. Web beryllium ores and concentrates 2617.90.0030 free. Web like all elements in the 2nd group on the periodic table, beryllium has +2 oxidation state. While beryl (4% beryllium) is the principal mineral mined for beryllium in the rest of the world. 13, triel bonds (old group iii) 158; Covalent bonds are formed when two atoms share electrons. Web for example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: Web beryllium & beryllium compounds (as be) related pages. Beryllium forms an oxide layer making it not react with air or water even in extreme heat. In addition, eight criteria based on qtaim properties, including the values of electron density and its laplacian at the bcp, penetration of beryllium and acceptor atom, charge. Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. Noncombustible solid in bulk form, but a slight explosion hazard in the form of a powder or dust. Thickness of 5 millimeters (mm) or more 7409.90.1030 3.0% ad val. In a study published today in science, chemists from the university of oxford explain how they have prepared the first stable compound containing a chemical bond between two beryllium atoms. Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. Web the molecular graphs of the investigated complexes indicate that beryllium in beh2 and bef2 can form “beryllium bonds” with o, n and p atoms but not with halogens. Beryllium oxide and hydroxide 2825.90.1000 3.7% ad val. Ds1750000 (metal) dot id & guide. Values are given for typical.Diagram representation of the element beryllium Vector Image
Beryllium Electron Configuration Electron configuration, Electrons
Beryllium Art Print by Carlos Clarivan Atomic structure, Electron
Ionic Compounds
Ionic Bonds Mooramo
Beryllium Periodic Table and Atomic Properties
Beryllium Element With Reaction, Properties and Uses Periodic Table
beryllium Properties, Uses, & Facts Britannica
Beryllium Chloride Facts, Formula, Properties, Uses, Safety Data
How Can We Find A Beryllium Electron Configuration (Be)
Related Post: