How Do Nonmetals Tend To Form Bonds
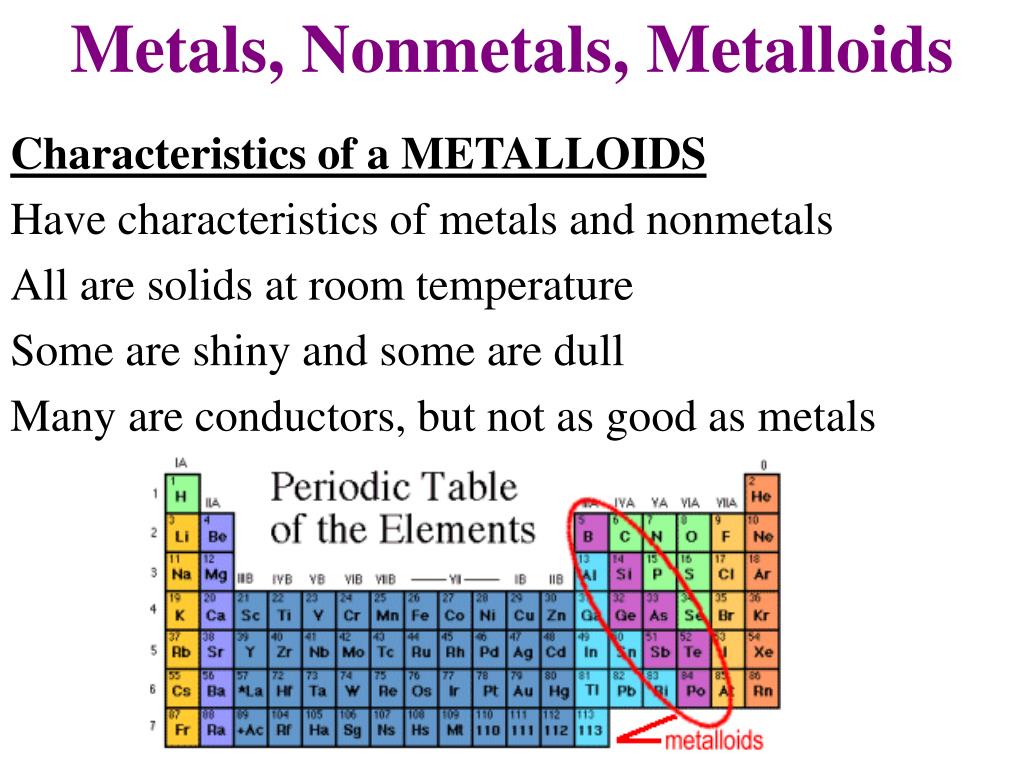
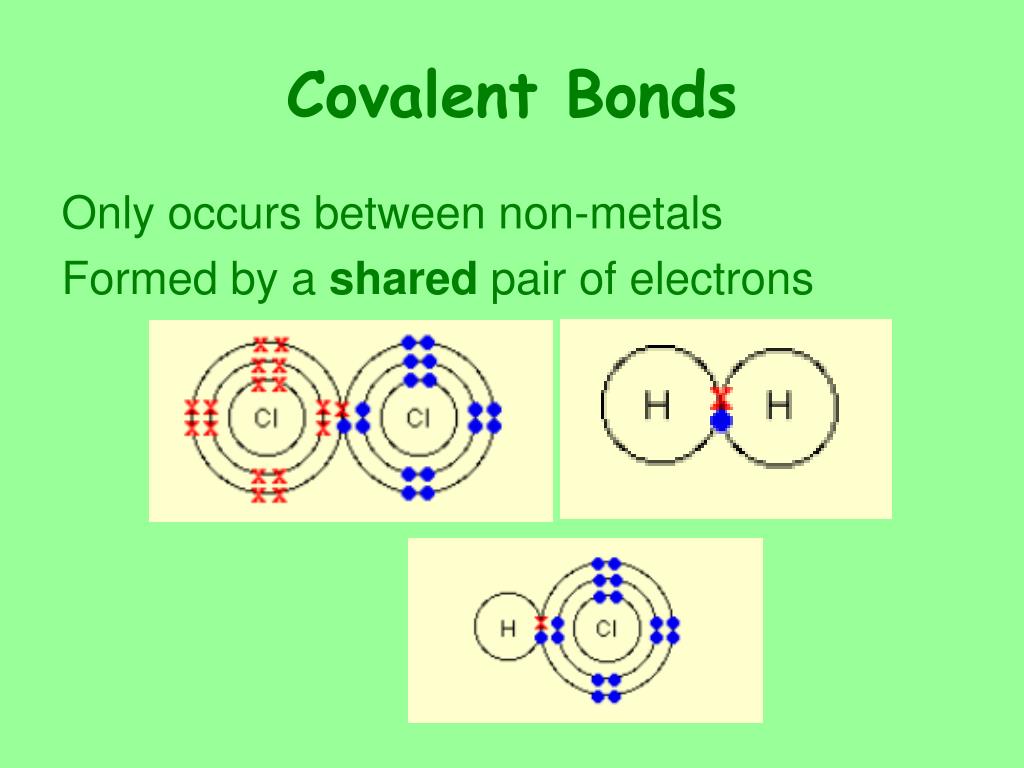
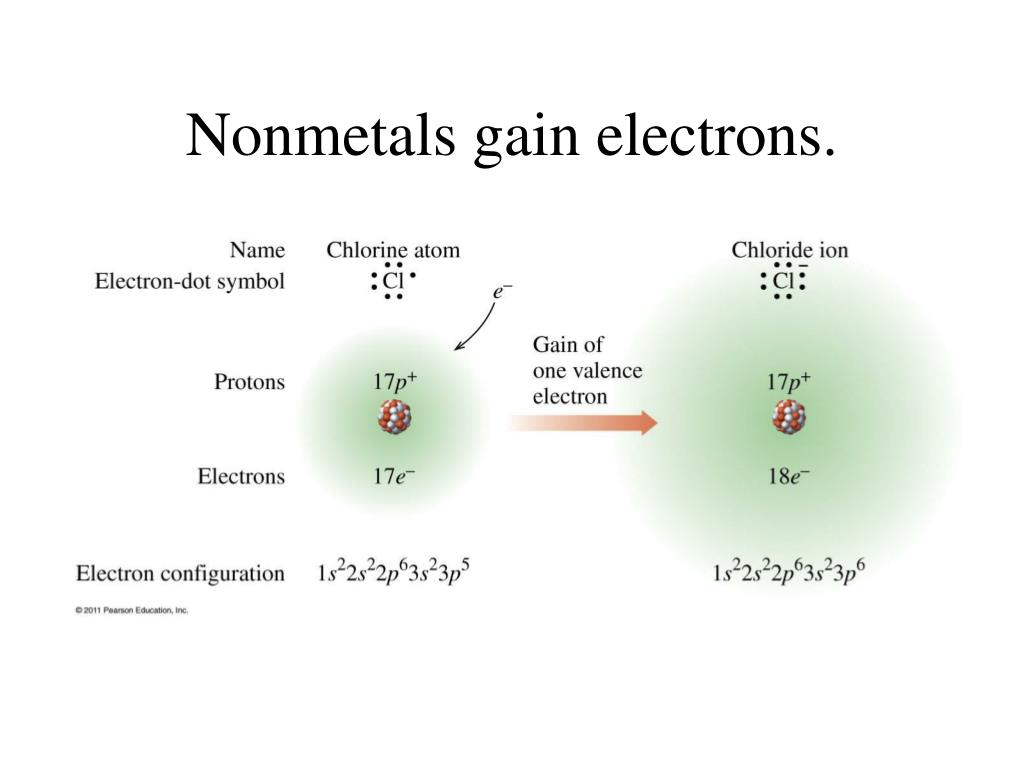
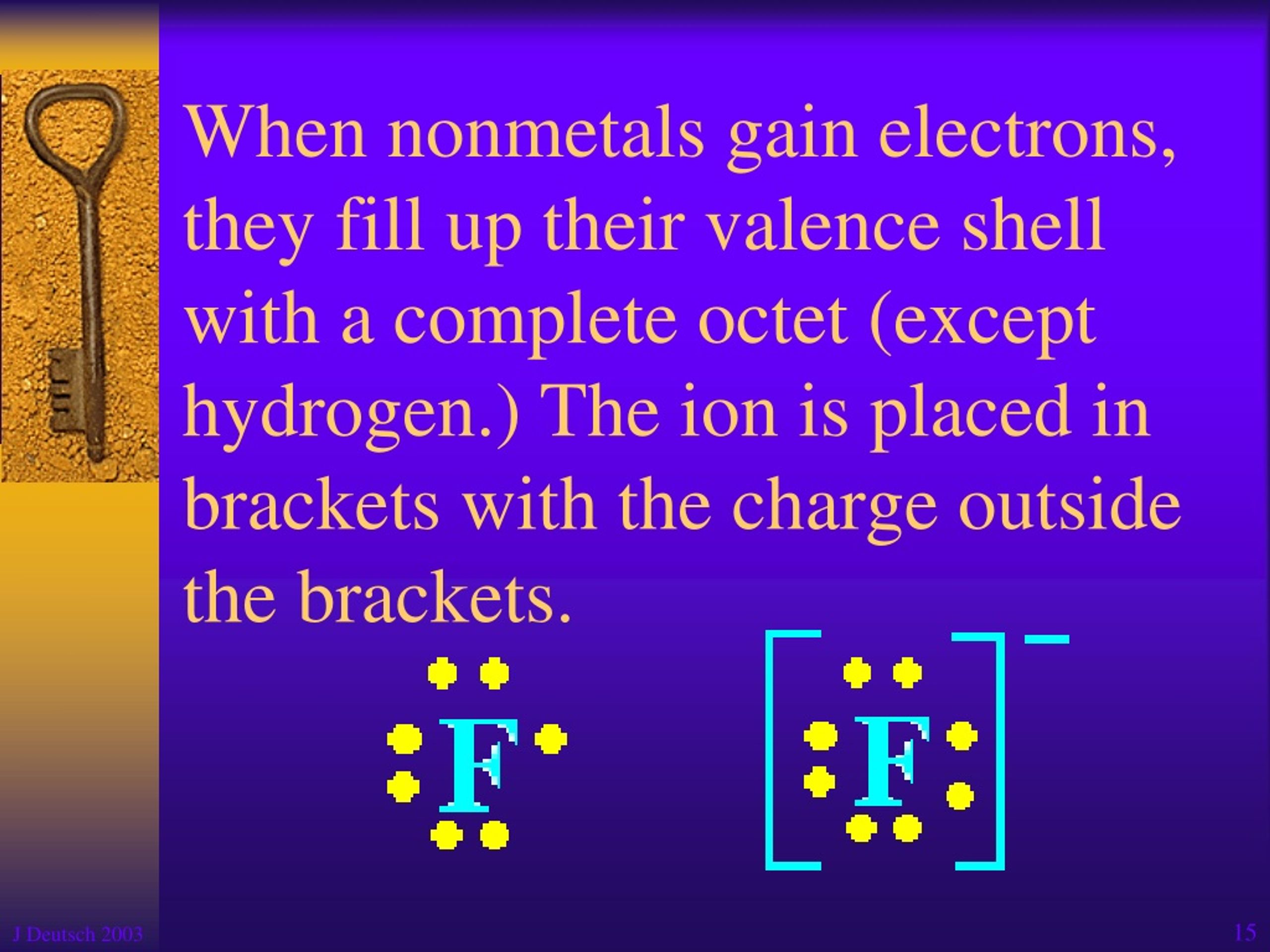
How Do Nonmetals Tend To Form Bonds - The have relatively high electron affinities and high ionization energies. Web which type of bond forms with nonmetals only? Web how do nonmetals tend to form bonds? These are electronegative elements with high. Web how do nonmetal atoms form bonds? For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Web when a chemical bond forms between metal and nonmetal atoms, the nonmetal atom accepts electrons from the metal atom to reach a stable state. Web thus, nonmetals tend to form negative ions. Web thus, nonmetals tend to form negative ions. Web thus, nonmetals tend to form negative ions. Web terms in this set (76) second period. Web thus, nonmetals tend to form negative ions. Web how do nonmetals tend to form bonds? Metal atoms lose electrons to form positive ions ( cations. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Only one more electron is. Metal atoms lose electrons to form positive ions ( cations. Positively charged ions are called cations, and negatively charged ions are called anions. Metals tend to lose electrons. These are electronegative elements with high. Web how do nonmetals tend to form bonds? Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Which feature is unique to group 18 nonmetals? Web thus, nonmetals tend to form negative ions. Positively charged ions are called cations, and negatively charged ions are called anions. The have relatively high electron affinities and high ionization energies. Metal atoms lose electrons to form positive ions ( cations. Web nonmetals, other than the first member of each group, rarely form π bonds to nonmetals that are the first member of a group. In general, covalent. Web most nonmetals become anions when they make ionic compounds. Positively charged ions are called cations, and negatively charged ions are called anions. Web thus, nonmetals tend to form negative ions. Web which type of bond forms with nonmetals only? Ions can be either monatomic (containing only. These are electronegative elements with high. Web nonmetals, other than the first member of each group, rarely form π bonds to nonmetals that are the first member of a group. Web most nonmetals become anions when they make ionic compounds. Nonmetal atoms form bonds by gaining or sharing valence electrons. For example, the hydrogen molecule, h 2, contains a covalent. Positively charged ions are called cations, and negatively charged ions are called anions. Web how do nonmetal atoms form bonds? For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Web nonmetals, other than the first member of each group, rarely form π bonds to nonmetals that are the first member of a group.. Only one more electron is. Ions can be either monatomic (containing only. Web which type of bond forms with nonmetals only? Metal atoms lose electrons to form positive ions ( cations. Metals tend to lose electrons. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Web most nonmetals become anions when they make ionic compounds. Web thus, nonmetals tend to form negative ions. Web thus, nonmetals tend to form negative ions. For example, the hydrogen molecule, h 2, contains a covalent. Metal atoms lose electrons to form positive ions ( cations. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Web nonmetals, other than the first member of each group,. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Web thus, nonmetals tend to form negative ions. Web nonmetals, other than the first member of each group, rarely form π bonds to nonmetals that are the first member of a group. Positively charged ions are called cations, and negatively charged ions are called anions. Which of the images represents the metallic crystal structure. Metal atoms lose electrons to form positive ions ( cations. The have relatively high electron affinities and high ionization energies. Ions can be either monatomic (containing only. Web how do nonmetals tend to form bonds? Positively charged ions are called cations, and negatively charged ions are called anions. Which feature is unique to group 18 nonmetals? Metals tend to lose electrons. Web most nonmetals become anions when they make ionic compounds. Web how do nonmetal atoms form bonds? Web when a chemical bond forms between metal and nonmetal atoms, the nonmetal atom accepts electrons from the metal atom to reach a stable state. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. Web which type of bond forms with nonmetals only? Nonmetal atoms form bonds by gaining or sharing valence electrons. Only one more electron is. Web thus, nonmetals tend to form negative ions.Metals Nonmetals And Metalloids Chart
PPT Covalent Bonding PowerPoint Presentation, free download ID5648526
PPT Nomenclature of Compounds PowerPoint Presentation, free
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609
Ionic Bond Definition, Types, Properties & Examples
How does a polar bond differ from a covalent bond
Covalent Bonding Between Two Non Metals (Atomic Bonding Mechanisms
PPT Unit 14 Chemical Bonding PowerPoint Presentation, free download
Related Post: