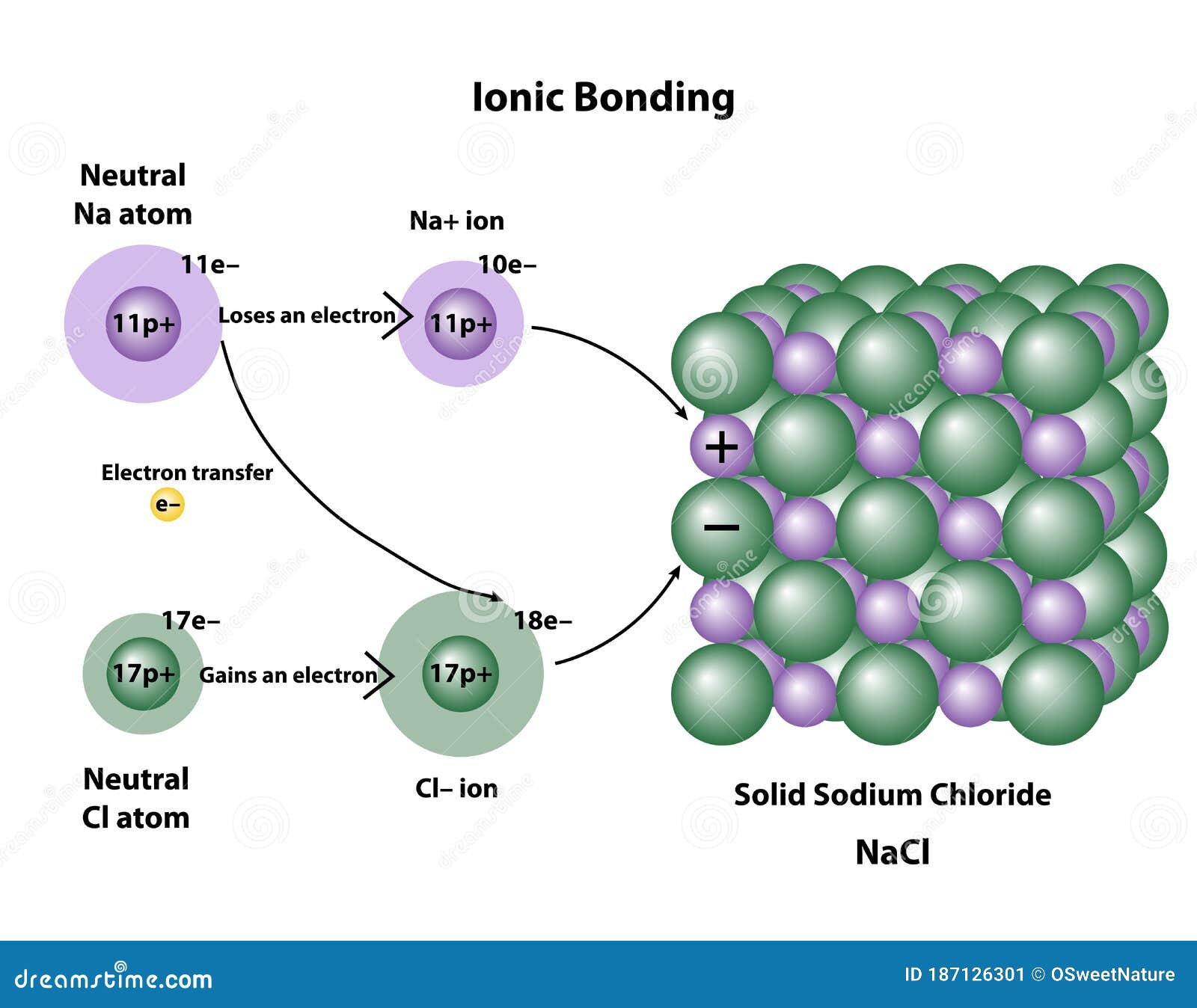
How Do Ionic Compounds Form Crystals
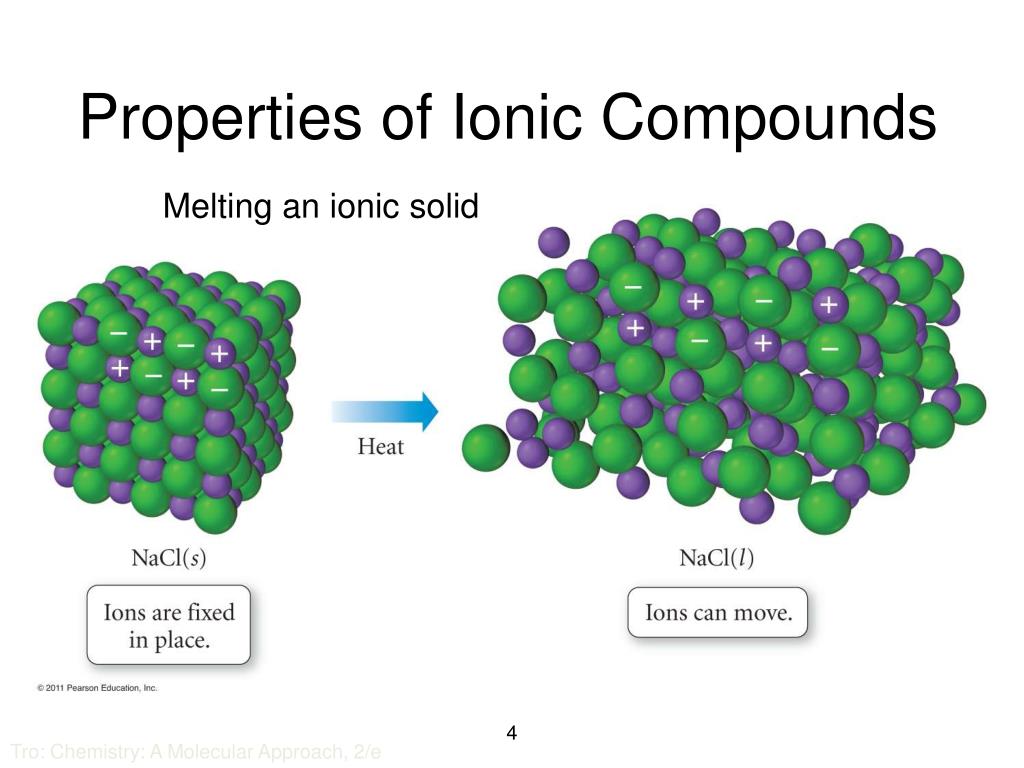
How Do Ionic Compounds Form Crystals - Web a certain number of anions encircle each cation and vice versa. They are solids consisting of ions bound together by their electrostatic attraction into a regular. Web in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Most of the rocks and minerals that surround us are made of ions held together through ionic bonding, the electrical attraction between. Web a crystal forms to balance the forces of repulsion and attraction amongst the ions. The electrostatic forces of attraction between oppositely. Web ionic compounds have high boiling and melting points as they’re very strong and require a lot of energy to break. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. The solubility of ionic compounds. Web you can get into further detail about ionic solids by inspecting the ions’ size or the type of crystal structure they adopt to maximize attractions, but the fundamental. Web a crystal forms to balance the forces of repulsion and attraction amongst the ions. Web ionic compounds have high boiling and melting points as they’re very strong and require a lot of energy to break. Web a certain number of anions encircle each cation and vice versa. Web in chemistry, an ionic crystal is a crystalline form of an. Web you can get into further detail about ionic solids by inspecting the ions’ size or the type of crystal structure they adopt to maximize attractions, but the fundamental. Web a crystal forms to balance the forces of repulsion and attraction amongst the ions. Web in the solid state, ionic compounds are in crystal lattice containing many ions each of. The precise pattern depends on the compound. Because of the strong electrostatic interaction between these oppositely charged ions, ionic. Web a certain number of anions encircle each cation and vice versa. Web ionic compounds have high boiling and melting points as they’re very strong and require a lot of energy to break. The crystals have ionic bonding, and each ion. The crystals have ionic bonding, and each ion has six or eight neighbours. Web ionic compounds have high boiling and melting points as they’re very strong and require a lot of energy to break. Metal ions in the alkaline earth series (magnesium [mg], calcium [ca],. The precise pattern depends on the compound. Web instead of amorphous solids, ionic compounds produce. Web in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Web instead of amorphous solids, ionic compounds produce crystal lattices. They are solids consisting of ions bound together by their electrostatic attraction into a regular. Though molecular substances may form crystals, they generally do so in different shapes than ionic.. The precise pattern depends on the compound. Web covalent compounds share electrons between two or more bonded atoms whereas metallic compounds essentially share electrons with all the other metal atoms in the compound. Though molecular substances may form crystals, they generally do so in different shapes than ionic. To classify solids as ionic, molecular, covalent (network), or. Although molecular compounds. Ionic compounds form crystal lattices rather than amorphous solids. To understand the correlation between bonding and the properties of solids. Web ionic compounds have high boiling and melting points as they’re very strong and require a lot of energy to break. Because of the strong electrostatic interaction between these oppositely charged ions, ionic. The solubility of ionic compounds. To classify solids as ionic, molecular, covalent (network), or. Because of the strong electrostatic interaction between these oppositely charged ions, ionic. An ionic formula, like nacl nacl, is an empirical. Web a crystal forms to balance the forces of repulsion and attraction amongst the ions. To understand the correlation between bonding and the properties of solids. Because of the strong electrostatic interaction between these oppositely charged ions, ionic. Web you can get into further detail about ionic solids by inspecting the ions’ size or the type of crystal structure they adopt to maximize attractions, but the fundamental. They are solids consisting of ions bound together by their electrostatic attraction into a regular. The precise pattern depends. Web a certain number of anions encircle each cation and vice versa. Web instead of amorphous solids, ionic compounds produce crystal lattices. Ionic compounds form crystal lattices rather than amorphous solids. The crystals have ionic bonding, and each ion has six or eight neighbours. To classify solids as ionic, molecular, covalent (network), or. Web covalent compounds share electrons between two or more bonded atoms whereas metallic compounds essentially share electrons with all the other metal atoms in the compound. The precise pattern depends on the compound. Web ionic compounds have high boiling and melting points as they’re very strong and require a lot of energy to break. The arrangement maximizes the attractive force between. The lattice is formed because the ions attract each other and form a regular pattern with oppositely charged ions next to each other. Web instead of amorphous solids, ionic compounds produce crystal lattices. The solubility of ionic compounds. Though molecular substances may form crystals, they generally do so in different shapes than ionic. Although molecular compounds form crystals, they frequently take other forms plus. Because of the strong electrostatic interaction between these oppositely charged ions, ionic. Some ionic compounds form different structures depending on how the are solidified. Web you can get into further detail about ionic solids by inspecting the ions’ size or the type of crystal structure they adopt to maximize attractions, but the fundamental. An ionic formula, like nacl nacl, is an empirical. Web in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. To understand the correlation between bonding and the properties of solids. Web a certain number of anions encircle each cation and vice versa. Ionic compounds form crystal lattices rather than amorphous solids. Metal ions in the alkaline earth series (magnesium [mg], calcium [ca],. The crystals have ionic bonding, and each ion has six or eight neighbours. Web a crystal forms to balance the forces of repulsion and attraction amongst the ions.Properties of ionic compounds Chemistry Quizizz
Ionic Solids Chemistry LibreTexts
What are Ionic Compounds and how they are formed?
Ionic Bonding Presentation Chemistry
PPT Ionic Bonding Part I PowerPoint Presentation, free download ID
PPT Ionic Bonds PowerPoint Presentation, free download ID1990542
What Is An Ionic Compound? Formula and Defination
Ionic Bonding Presentation Chemistry
PPT Introduction to Ionic Compounds PowerPoint Presentation ID690533
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector
Related Post:
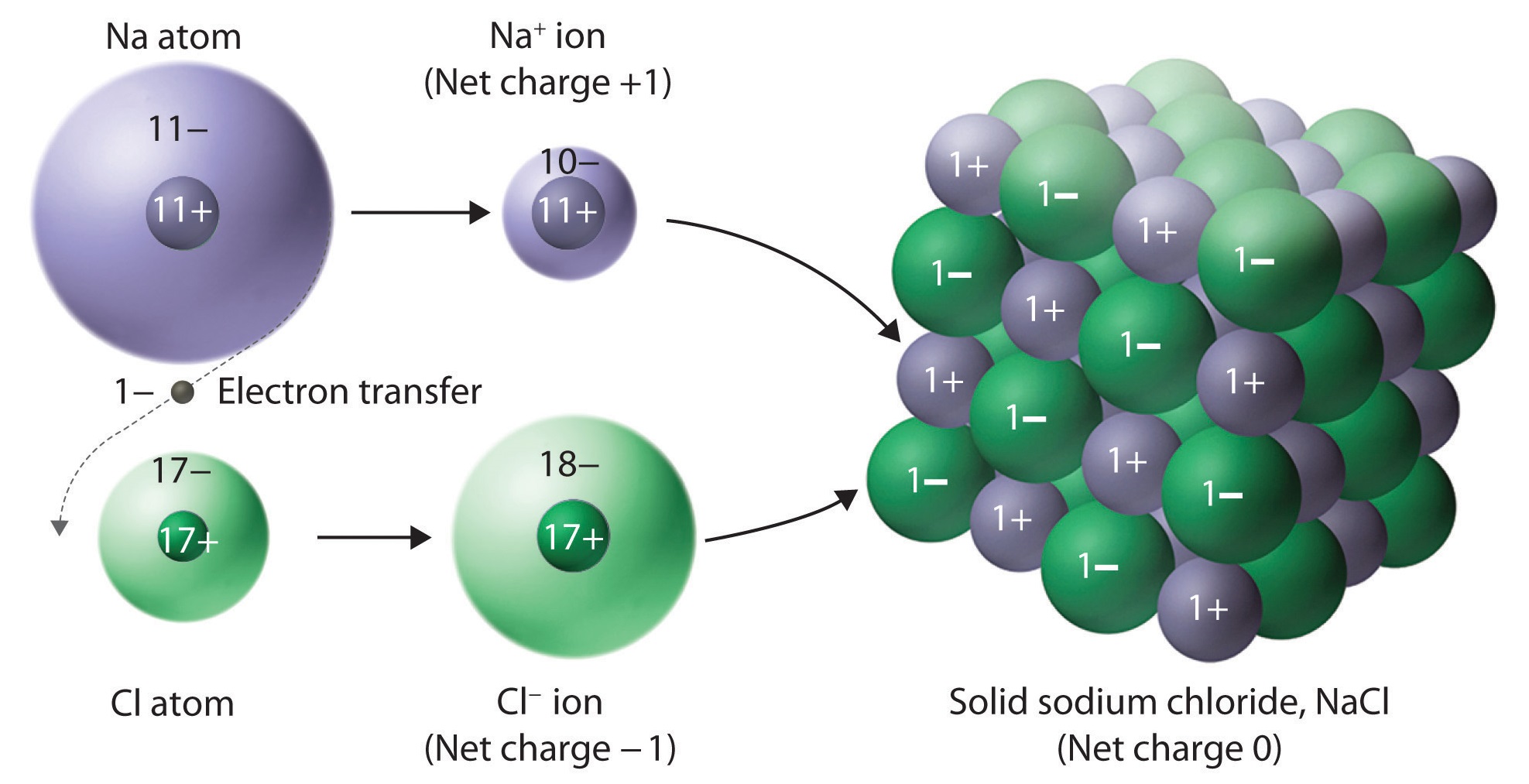

.PNG)
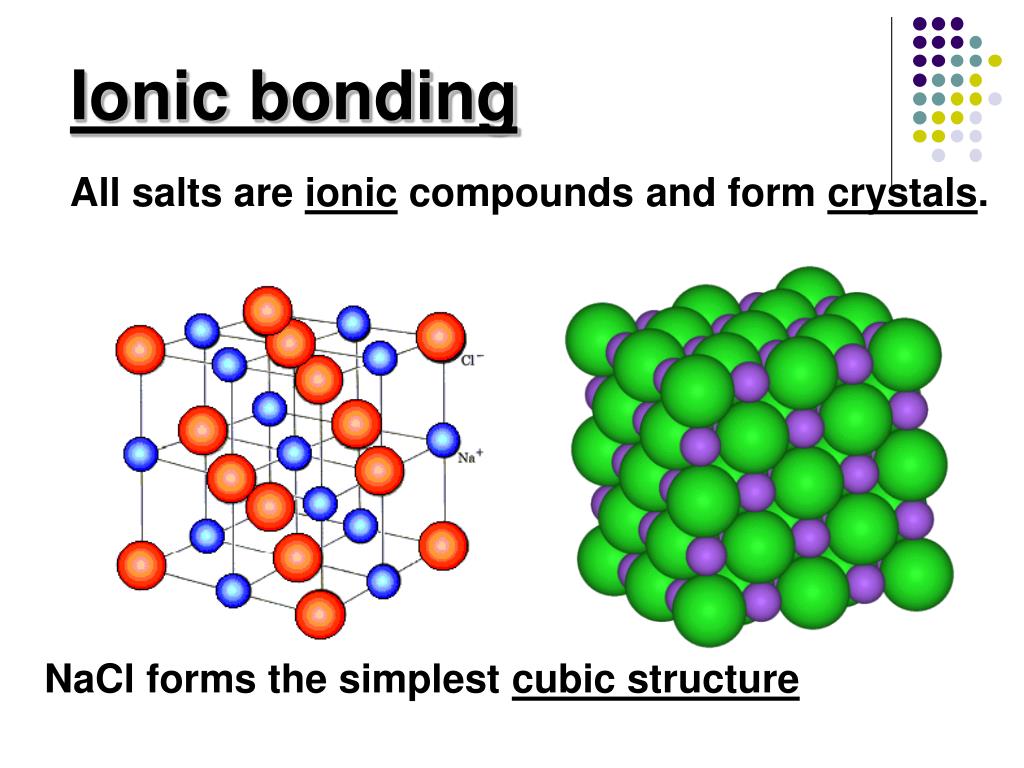


.PNG)