How Do Atoms Form Chemical Bonds
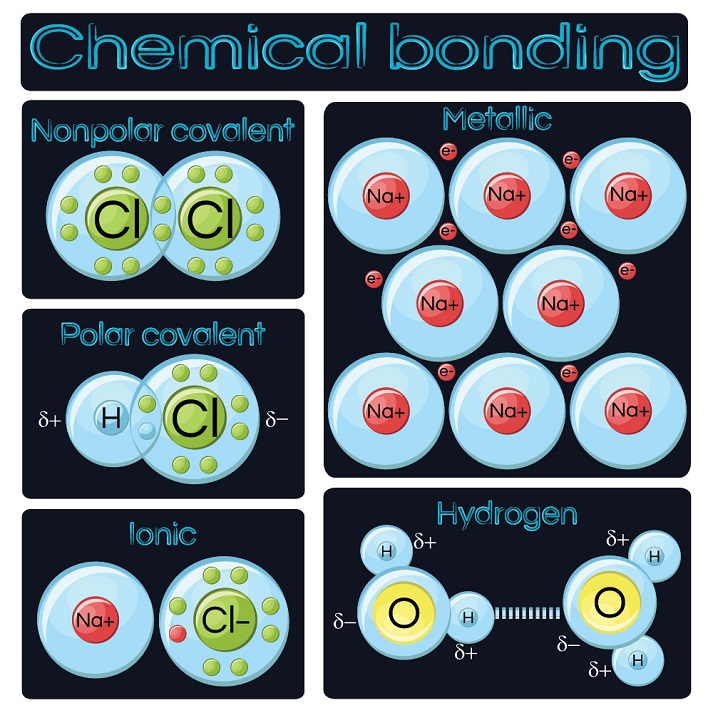
How Do Atoms Form Chemical Bonds - Chemical bonds (exercises) these are exercises and select solutions to accompany chapter 9 of the beginning chemistry textmap formulated around the ball et al. Web there are three basic ways that the outer electrons of atoms can form bonds: Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions. There are three different types of. Web the process of reorganizing atoms by breaking one set of chemical bonds and forming a new set is known as a chemical reaction. The columns of the periodic table, which contain elements that show a family resemblance, are called groups. Web chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. Web why form chemical bonds? The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms. Atomic structure of carbon atom showing the particles of an atom: Web chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. Web chemical bonds form when the valence electrons of one atom interact with the valence electrons of another atom. Web why form chemical bonds? As we will now see, there are a variety of different ways to represent and draw. Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. [a note on definitions] representing molecules: Web there are three basic ways that the outer electrons of atoms can form bonds: Valence electrons are the electrons in the outer energy level of an atom that may be involved in. There are three different types of. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. Web atom, smallest unit into which matter can be divided without the release of electrically charged particles. Chemical bonds are formed when electrons in different atoms interact with. How elements interact with one another depends on how their electrons are arranged and how many openings for electrons exist at the outermost region where electrons are present in an atom. Is determined by the distance at which the lowest potential energy is achieved. As such, the atom is the basic building block of chemistry. Web when atoms combine by. As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The first way gives rise to what is called an ionic bond. Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions.. Three idealized types of bonding are ionic bonding , in which positively and negatively charged ions are held together by electrostatic forces, covalent bonding , in which electron pairs are shared between atoms and metallic. Web atom, smallest unit into which matter can be divided without the release of electrically charged particles. In a homonuclear molecule such as o2 the. Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. The first way gives rise to what is called an ionic bond. Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Web covalent bonding occurs when pairs of electrons. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when the atoms are apart. Let’s begin… atoms can (and do) bond constantly; Figure 1a shows a protein, depicted in green, attached to a dna molecule, shown in orange. Chemical bonds (exercises) these are exercises and select solutions. Therefore, the valence electrons have the most influence in. Web why form chemical bonds? Three idealized types of bonding are ionic bonding , in which positively and negatively charged ions are held together by electrostatic forces, covalent bonding , in which electron pairs are shared between atoms and metallic. Chemical bonds (exercises) these are exercises and select solutions to accompany. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. As such, the atom is the basic building block of chemistry. Consider as an example an atom of sodium, which has one electron in its outermost orbit, coming near an atom of chlorine, which. Web why form chemical bonds? Web the process of reorganizing atoms by breaking one set of chemical bonds and forming a new set is known as a chemical reaction. When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement. We can therefore say that a molecule is the simplest unit of a covalent compound. The columns of the periodic table, which contain elements that show a family resemblance, are called groups. Atomic structure of carbon atom showing the particles of an atom: Web chemical bonds form when the valence electrons of one atom interact with the valence electrons of another atom. Chemical bonds (exercises) these are exercises and select solutions to accompany chapter 9 of the beginning chemistry textmap formulated around the ball et al. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The first way gives rise to what is called an ionic bond. How elements interact with one another depends on how their electrons are arranged and how many openings for electrons exist at the outermost region where electrons are present in an atom. Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. It's how they form molecules. Bonds form when atoms share or transfer valence electrons. Valence electrons are the basis of all chemical bonds. Web covalent bonding occurs when pairs of electrons are shared by atoms. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. Atoms can also play nicely and share electrons in a covalent bond. Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions.Chemistry B.Sc Level How many types of chemical bond
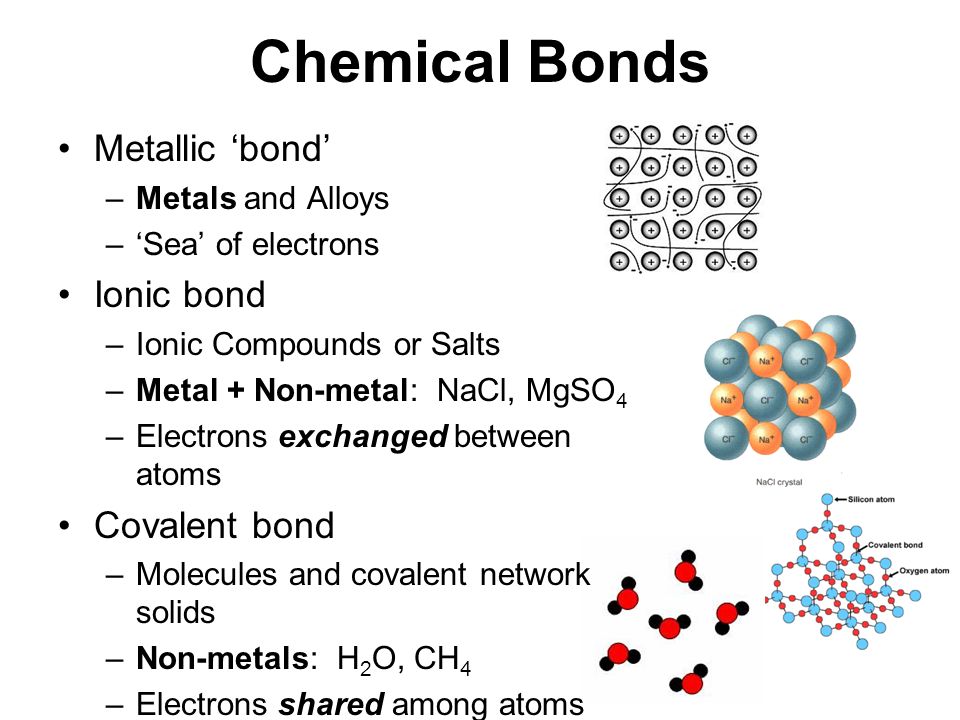
Metallic Chemical Bonds Educational Resources K12 Learning, Chemistry
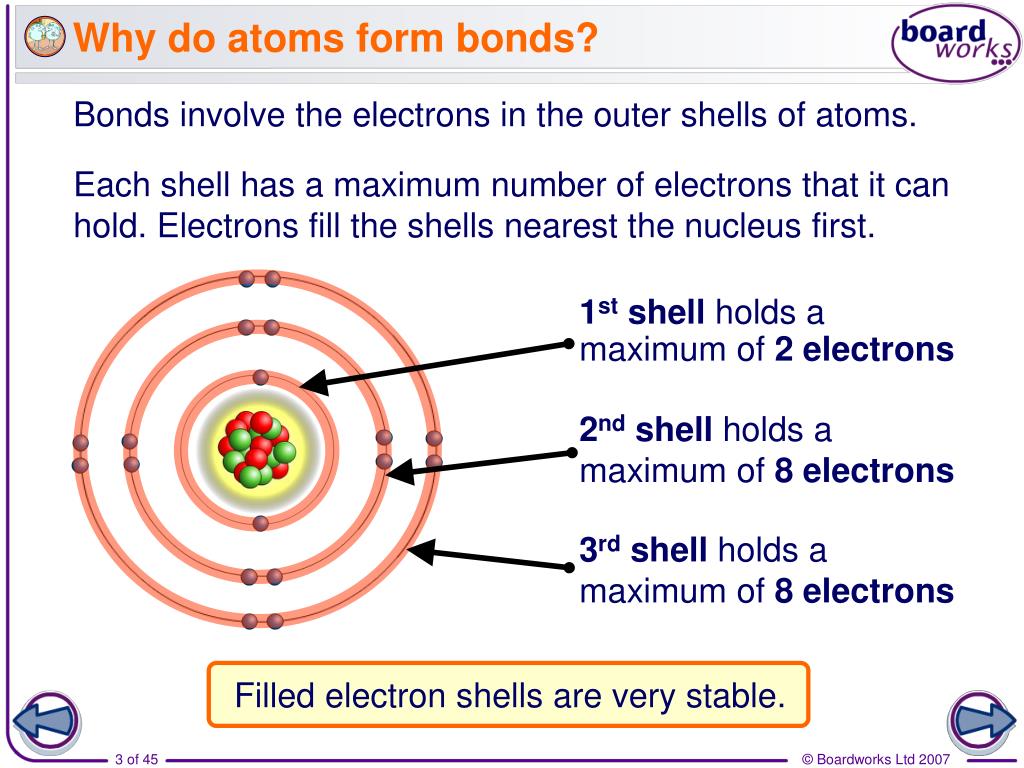
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Covalent bond Definition, Properties, Examples, & Facts Britannica
ionic bond Definition, Properties, Examples, & Facts Britannica
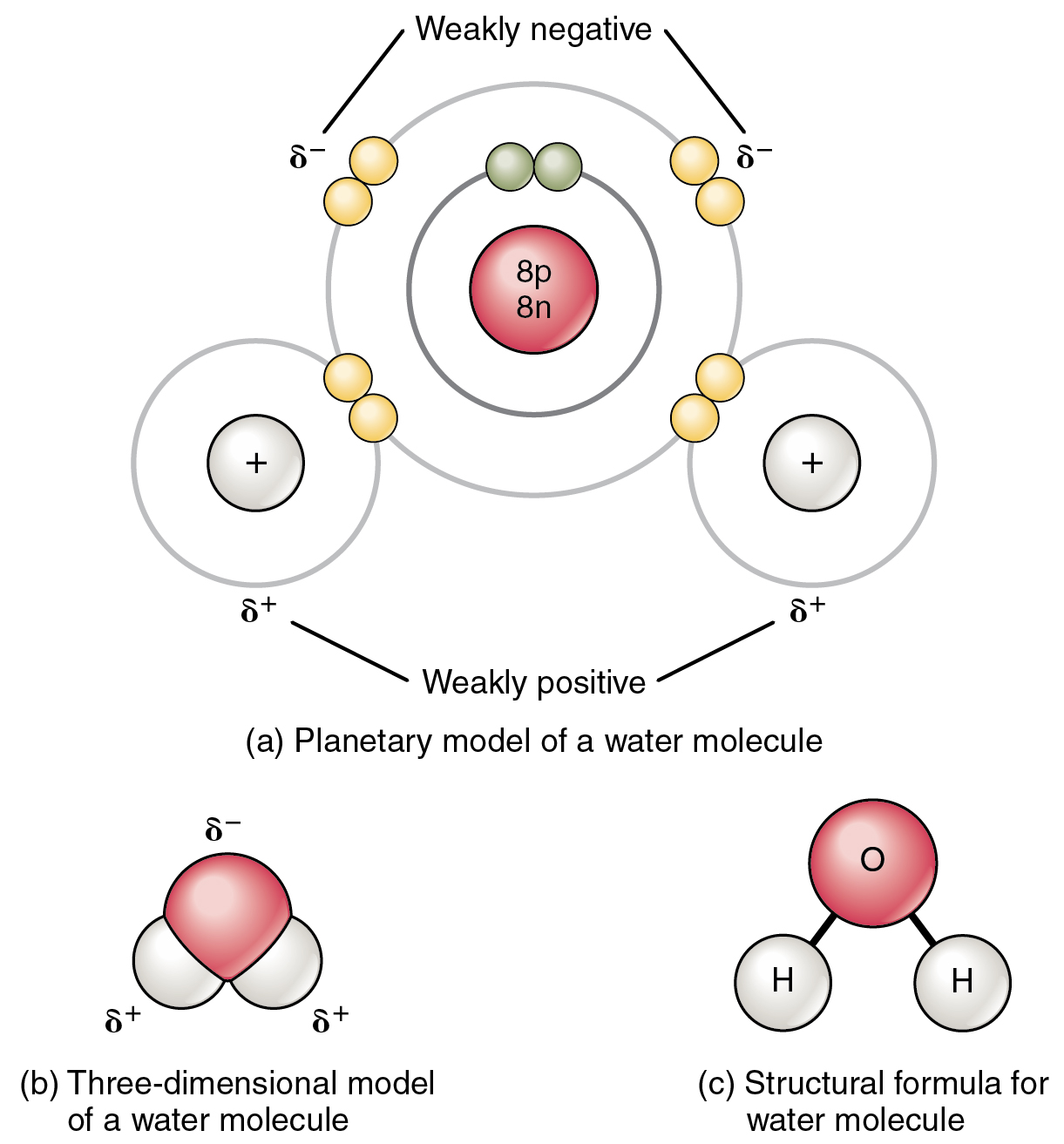
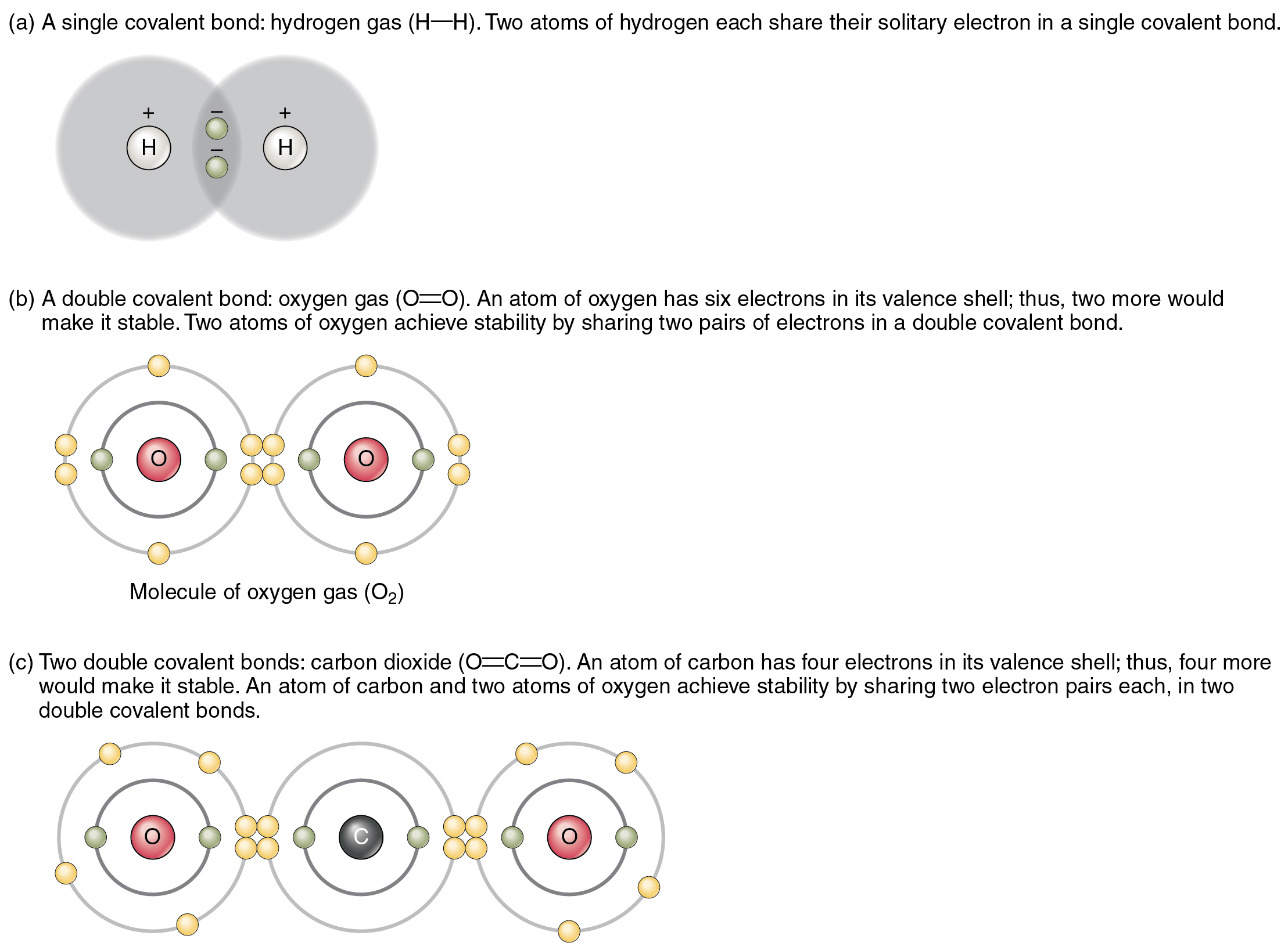
Chemical Bonds · Anatomy and Physiology
Ionic Bond Definition, Types, Properties & Examples
chemical bonding Definition, Types, & Examples Britannica
What Happens When Atoms Bond infographic diagram showing how electrons
Chemical Bonds · Anatomy and Physiology
Related Post: