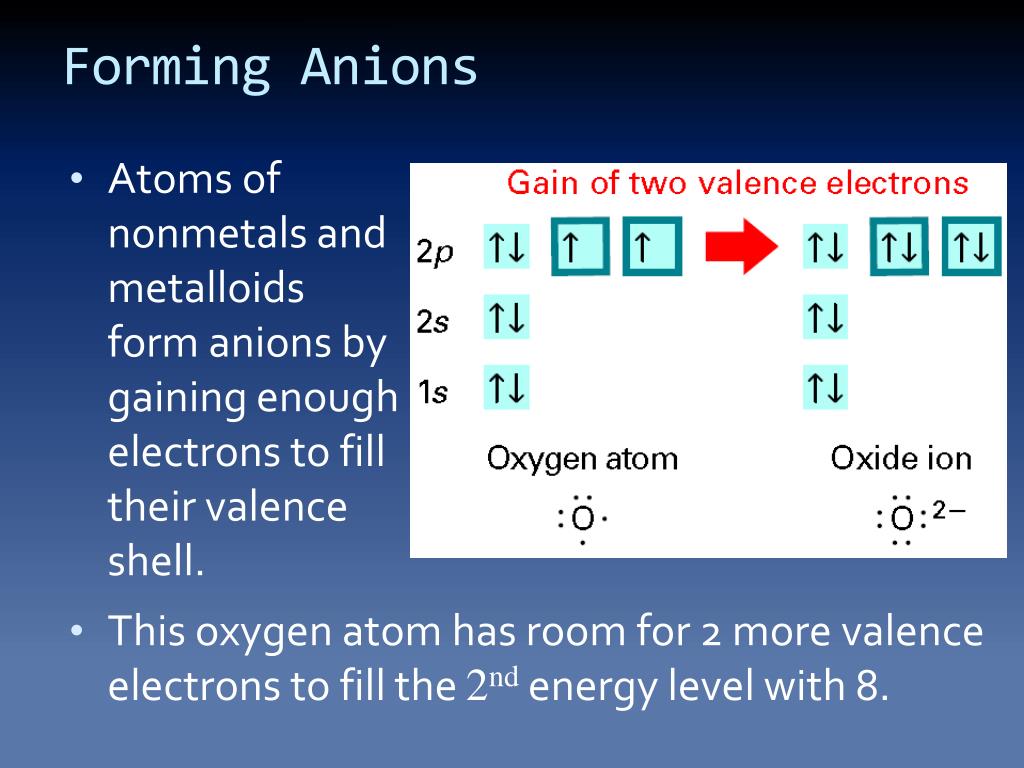
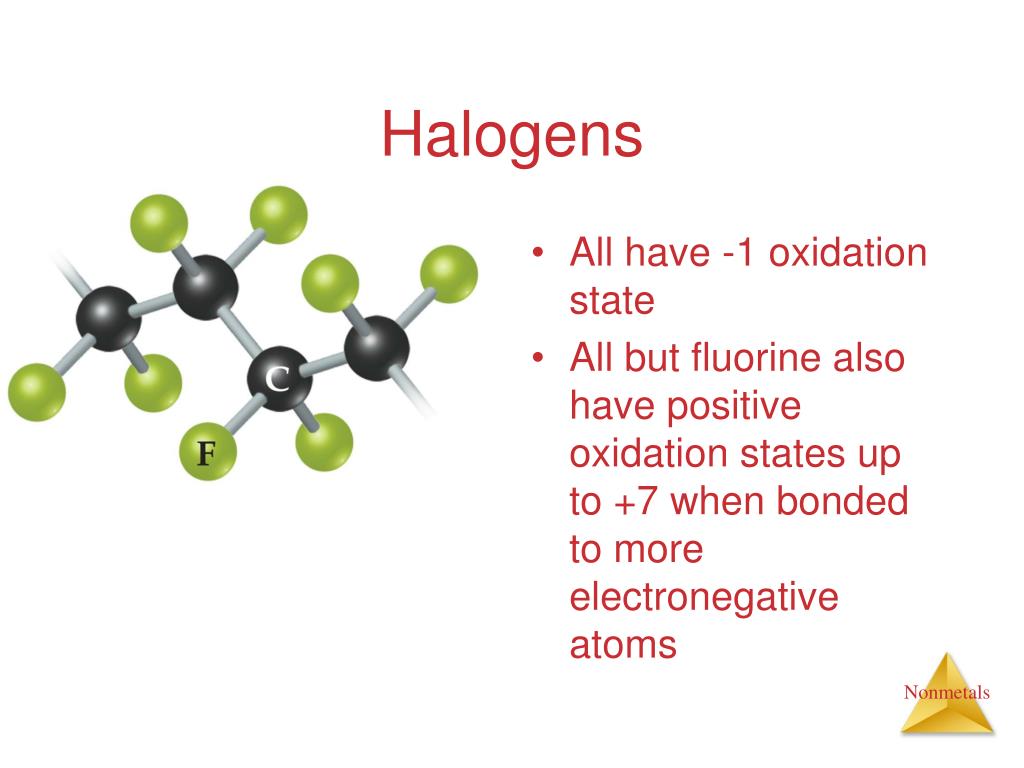
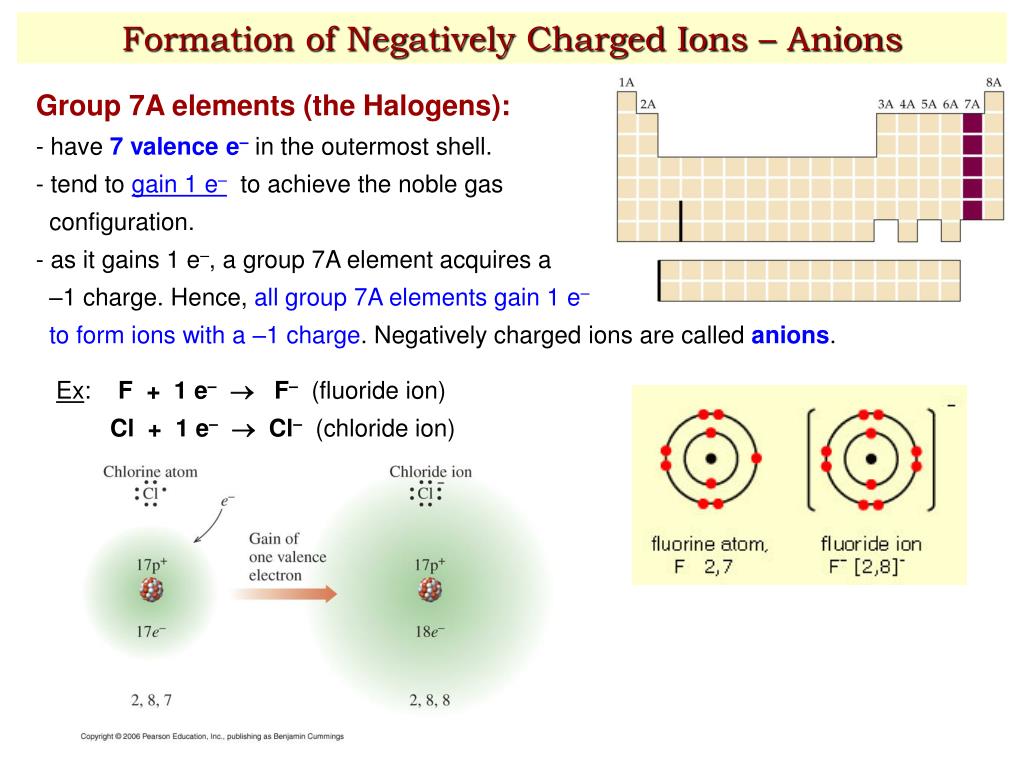
Halogens Tend To Form Anions Because
Halogens Tend To Form Anions Because - Web the halogens all have relatively high ionization energies, but the energy required to remove electrons decreases substantially as we go down the column. You may also hear charges. B) gaining electrons will fill their octet faster than losing. Web role of halogens in solid electrolytes. Halogens tend to form anions, which are negative ions that form by gaining electrons. 1) they give up one electron to achieve the octet rule. Web we would like to show you a description here but the site won’t allow us. Halogens tend to form anions because a) losing electrons will make them attain a noble gas configuration faster than gaining them. Ions made from alkaline earth metals, the second group on the. Web for example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. This is due to the fact that gaining electrons will fill their octet. 2) have 7 valence electrons and need one more which. Since they are reduced when this occurs,. Web science chemistry halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. As mentioned in the previous section, the application of halogens. B) gaining electrons will fill their octet faster than losing. Halogens tend to form anions, which are negative ions that form by gaining electrons. Since they are reduced when this occurs,. Web all halogens form group 1 salts with similar properties. Web we would like to show you a description here but the site won’t allow us. As mentioned in the previous section, the application of halogens in ses started with lix (where x = f, cl, br, i). 2) have 7 valence electrons and need one more which. Web science chemistry halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. Web the halogens all have relatively high ionization. Web role of halogens in solid electrolytes. The halogens tend to form anions because. Thus, the electron affinity will be. As expected, these elements have certain properties in common. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); 2) have 7 valence electrons and need one more which. B) gaining electrons will make them. As expected, these elements have certain properties in common. As mentioned in the previous section, the application of halogens in ses started with lix (where x = f, cl, br, i). Web all halogens form group 1 salts with similar properties. B) gaining electrons will make them. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web the halogens tend to form anions because. Web why do halogens tend to form anions? Web the halogens all have relatively high ionization energies, but the energy required to remove electrons. The halogens tend to form anions because. Web why do halogens tend to form anions? Ions made from alkaline earth metals, the second group on the. Web all halogens form group 1 salts with similar properties. Web science chemistry halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. Halogens tend to form anions, which are negative ions that form by gaining electrons. 2) have 7 valence electrons and need one more which. The halogens tend to form anions because. As expected, these elements have certain properties in common. Web role of halogens in solid electrolytes. B) gaining electrons will fill their octet faster than losing. Ions made from alkaline earth metals, the second group on the. You may also hear charges. 1) they give up one electron to achieve the octet rule. Halogens tend to form anions, which are negative ions that form by gaining electrons. Web for example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. You may also hear charges. Halogens tend to form anions, which are negative ions that form by gaining electrons. This is due to the fact that gaining electrons will fill their octet. Web all halogens form group 1 salts with. Web science chemistry halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. Ions made from alkaline earth metals, the second group on the. Web for example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Halogens tend to form anions, which are negative ions that form by gaining electrons. Web the halogens all have relatively high ionization energies, but the energy required to remove electrons decreases substantially as we go down the column. This is due to the fact that gaining electrons will fill their octet. Web the halogens tend to form anions because. 1) they give up one electron to achieve the octet rule. B) gaining electrons will fill their octet faster than losing. Web all halogens form group 1 salts with similar properties. Since they are reduced when this occurs,. B) gaining electrons will make them. Web role of halogens in solid electrolytes. The halogens tend to form anions because. Solution verified answered 5 months ago create an account to view solutions recommended textbook solutions chemistry: Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); 2) have 7 valence electrons and need one more which. Thus, the electron affinity will be. You may also hear charges. As mentioned in the previous section, the application of halogens in ses started with lix (where x = f, cl, br, i).PPT IONS PowerPoint Presentation, free download ID2435906
Solved In which set do all elements tend to form anions in
PPT IONS PowerPoint Presentation, free download ID2435906
Cations and Anions Definitions, Examples, and Differences
Halogens tend to form anions because A) losing
Formation of anionPropertiesChemistry YouTube
PPT Chapter 22 Chemistry of the Nonmetals PowerPoint Presentation
Cations and Anions List KarsyntinOconnell
PPT 1 Name the ions formed by these elements and classify them as
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation
Related Post: