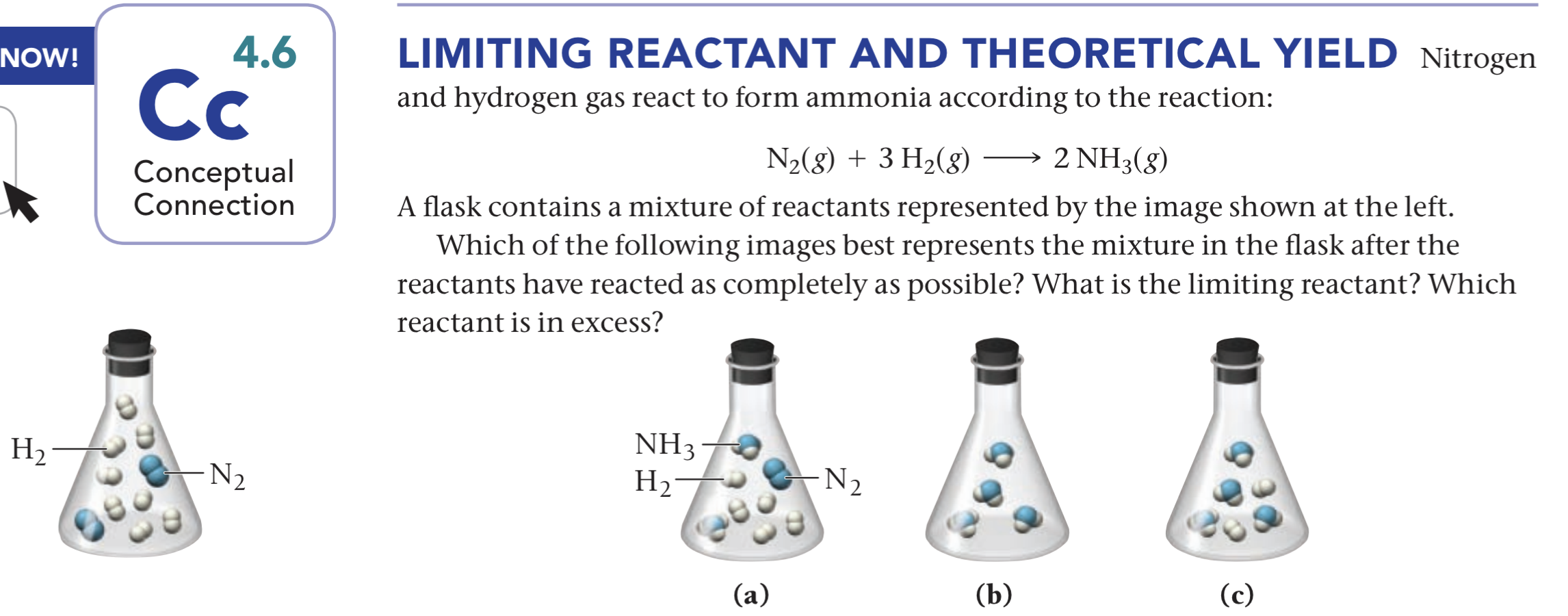
Gaseous Nitrogen And Hydrogen React To Form Gaseous Ammonia
Gaseous Nitrogen And Hydrogen React To Form Gaseous Ammonia - Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web the combustion of ammonia proceeds with difficulty but yields nitrogen gas and water. Calculate the largest amount of. 4nh 3 + 3o 2 + heat → 2n 2 + 6h 2 o however, with the use of a catalyst. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Hydrogen gas and nitrogen gas react to form ammonia gas. Suppose you have 13.0 mol of n2 and 1.0 mol of h2 in a reactor. Elementally, it reacts most commonly with. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. The reaction mixture contains some ammonia, plus a lot of unreacted nitrogen and hydrogen. N 2 ( g) + 3 h 2 ( g) → 2 nh 3 ( g) if all the n 2 and h 2 are consumed, what volume of nh 3 , at. Web nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. I mean to say that, when hydrogen (g) and nitrogen (g) combines together. Web nitrogen and hydrogen react to form ammonia gas according to the following unbalanced equation: Sarah was working in the lab, and she mixes ammonia with chlorine gas and gets nitrogen gas and hydrogen chloride gas. What volume of ammonia would be produced by this reaction if 6.9 m3 of nitrogen were consumed? Web nitrogen and hydrogen gases react to. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. The reaction mixture contains some ammonia, plus a lot of unreacted. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web the combustion of ammonia proceeds with difficulty but yields nitrogen gas and water. At a certain temperature and pressure, 1.2 l of n2 reacts with. Web nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. The reaction between the hydrogen gas. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Sarah was working in the lab, and she mixes ammonia with chlorine gas and gets nitrogen gas and hydrogen chloride gas. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. The reaction between the hydrogen gas. Web the following chemical reaction. The chemical equation is given as:. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web chemistry questions and answers. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Web if a reaction just involves gases, then the coefficients can be used to show relationships between moles of reactant and products and also leaders of reactant and products,. The reaction mixture contains some ammonia, plus a lot of unreacted nitrogen and hydrogen. At a certain temperature and pressure, 1.2 l of n2 reacts with. Web to calculate the number. The reaction mixture contains some ammonia, plus a lot of unreacted nitrogen and hydrogen. Elementally, it reacts most commonly with. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: 4nh 3 + 3o 2. Web nitrogen + hydrogen ⇌ ammonia. Web by the haber process, hydrogen gas reacts with nitrogen gas to produce ammonia. N21g2 + 3 h21g2¡2 nh31g2. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; 2 nh3 (g) + 3 cl2. Web by the haber process, hydrogen gas reacts with nitrogen gas to produce ammonia. Web nitrogen + hydrogen ⇌ ammonia. 2 nh3 (g) + 3 cl2. I mean to say that, when hydrogen (g) and nitrogen (g) combines together to form ammonia. Gaseous nitrogen and hydrogen react to form gaseous. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 (. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: The reaction between the hydrogen gas. I mean to say that, when hydrogen (g) and nitrogen (g) combines together to form ammonia. Web the combustion of ammonia proceeds with difficulty but yields nitrogen gas and water. Web nitrogen and hydrogen react to form ammonia gas according to the following unbalanced equation: Write a balanced chemical equation based on the following description: Suppose you have 13.0 mol of n2 and 1.0 mol of h2 in a reactor. Web nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web chemistry questions and answers. The mixture is cooled and. Hydrogen gas and nitrogen gas react to form ammonia gas. At a certain temperature and pressure, 1.2 l of n2 reacts with. What volume of ammonia would be produced by this reaction if 6.9 m3 of nitrogen were consumed? N21g2 + 3 h21g2¡2 nh31g2. Web by the haber process, hydrogen gas reacts with nitrogen gas to produce ammonia. Web if a reaction just involves gases, then the coefficients can be used to show relationships between moles of reactant and products and also leaders of reactant and products,.Nitrogen gas and hydrogen gas combine to form gaseous ammonia
Nitrogen Gas Reacts With Hydrogen Gas To Form Ammonia Printable Form
⚗️Question 6 (1 point) Hydrogen gas and nitrogen gas can react to form
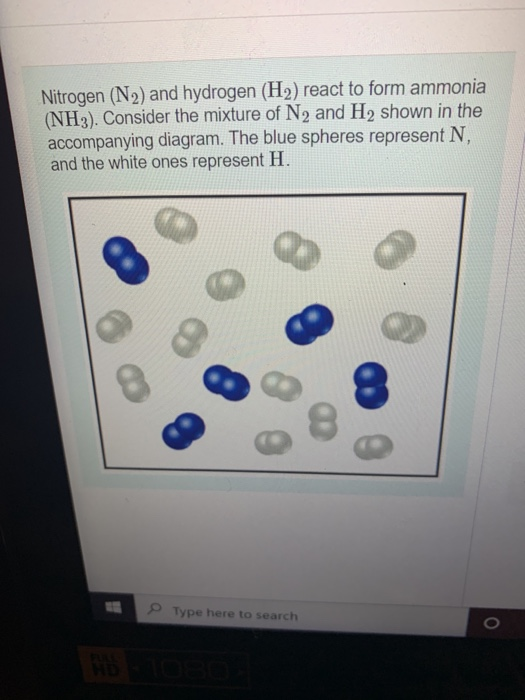
Solved Nitrogen (N2) and hydrogen (H2) react to form ammonia
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
[Solved] Hydrogen gas and nitrogen gas can react to form ammonia
Beautiful Work Nitrogen Reacts With Hydrogen Equation Of Respiration
⚗️Hydrogen gas and nitrogen gas can react to form ammonia according to
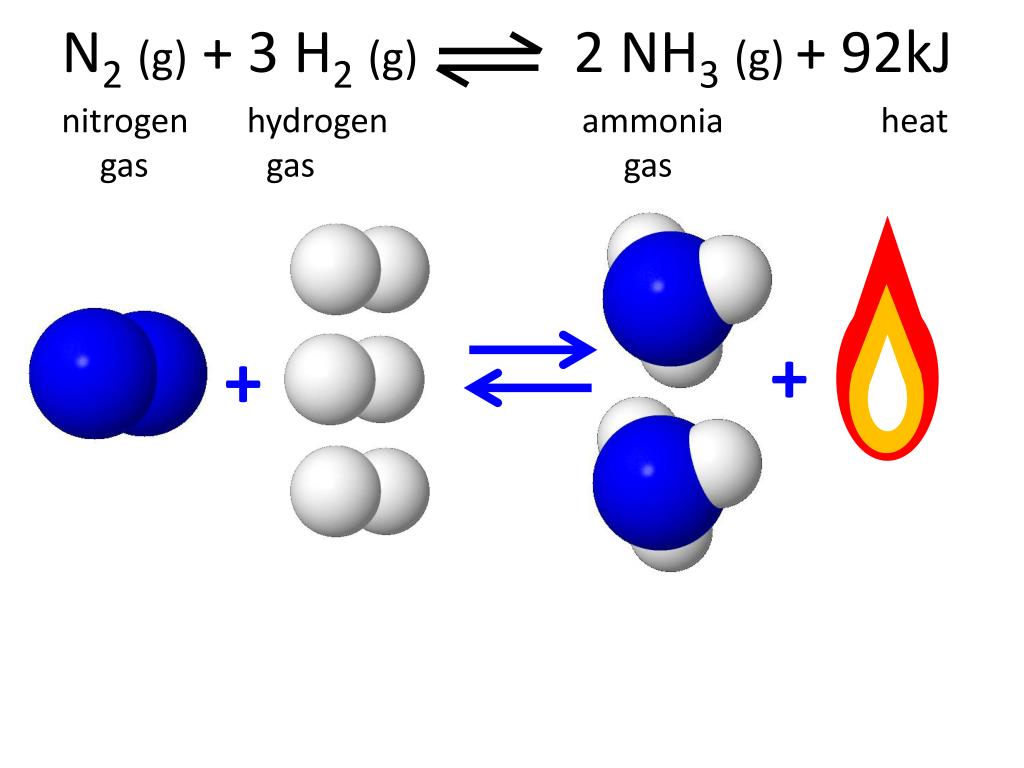
PPT Reaction Rates PowerPoint Presentation, free download ID6516140
Related Post: