Fda Form 483 Database
Fda Form 483 Database - Use the following instructions to download the form if. Web the form 483s database is an essential tool to prepare for your inspection. Web this document lists observations made by the fda representative(s) during the inspection of your facility. American red cross blood services, pomona, ca 483. Web form 483 to the management after the inspection, detailing the inappropriate conditions. Web the fda 483 can also be a prerequisite to an fda warning letter. Also referred to as form. Web inspectional records (eirs/483s) american red cross southeastern michigan region, detroit, mi 483 issued 9/27/2010. Redica systems has the world's largest fda 483 database aside from the fda itself. During this webinar, fda will provide an overview of what to expect after a compounding inspection. Web about this webinar. Web 11 rows for publicly available ora data sets, (such as lists of inspection classifications, 483 observations, etc.), please visit the data sets page. They’re literally just a click. Web who can see form fda 483s, and where do i get them? Web in a complex landscape that’s always in flux, the redicaid is a reliable. Web about this webinar. It's robust with thousands of downloadable 483s; You need to respond in. Citations data include form fda 483 citations and may not necessarily. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web the fda 483 can also be a prerequisite to an fda warning letter. Web form 483 to the management after the inspection, detailing the inappropriate conditions. Web form fda 483 (9/08. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web inspectional records (eirs/483s) american red cross southeastern michigan region, detroit, mi. *based on letter issue date [complis database as of feb 9, 2021]. American red cross blood services, pomona, ca 483. Also referred to as form. Redica systems july 5, 2018. The fda smacked samsung biologics for using technology to support application submission testing that had. As the keeper of the world’s largest database of fda 483s, we often get. Web fy 2022 ci 483 observation trends www.fda.gov. The fda 483 that is issued to the most responsible person at the company at the conclusion of an. Web form 483 to the management after the inspection, detailing the inappropriate conditions. Web fda 483 database form fda. As the keeper of the world’s largest database of fda 483s, we often get. Cfr reference | fdca reference. With a subscription to our form 483s database, you get instant, unlimited access to more than 8,600 form 483s. Because the fda guidelines are difficult to comply with, a company can contravene the. Also referred to as form. As the keeper of the world’s largest database of fda 483s, we often get. Cfr reference | fdca reference. Web who can see form fda 483s, and where do i get them? Depending on the browser you are using, you may need to download the form to enable field fillable functionality. They’re literally just a click. The fda 483 that is issued to the most responsible person at the company at the conclusion of an. Web form fda 483 (9/08. An fda form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that in their judgment may constitute. It’s robust with thousands of downloadable 483s. Also referred to as form. The fda smacked samsung biologics for using technology to support application submission testing that had. An fda form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that in their judgment may constitute. Redica systems has the world's largest fda 483 database aside from. With a subscription to our form 483s database, you get instant, unlimited access to more than 8,600 form 483s. The observations of objectionable conditions and. Redica systems july 5, 2018. Web who can see form fda 483s, and where do i get them? Cfr reference | fdca reference. It’s robust with thousands of downloadable 483s with more added each week. Drugs gmps inspections and audits. Web form fda 483 final classification (nai, vai, oai) letter. Redica systems july 5, 2018. Citations data include form fda 483 citations and may not necessarily. Web form 483 to the management after the inspection, detailing the inappropriate conditions. An fda form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that in their judgment may constitute. They’re literally just a click. Use the following instructions to download the form if. Web fda 483 database form fda 483 (or just 483s in industry shorthand) is the official inspection report that documents fda inspector observations of manufacturing facilities. Web about this webinar. The observations of objectionable conditions and. Web fy 2022 ci 483 observation trends www.fda.gov. Web form fda 483 (9/08. Web this document lists observations made by the fda representative(s) during the inspection of your facility. During this webinar, fda will provide an overview of what to expect after a compounding inspection. Web inspectional observations reflect data pulled from fda's electronic inspection tools. Web 11 rows for publicly available ora data sets, (such as lists of inspection classifications, 483 observations, etc.), please visit the data sets page. Redica systems has the world's largest fda 483 database aside from the fda itself. Web the form 483s database is an essential tool to prepare for your inspection.FDA 483 OBSERVATIONS An FDA Consulting Firm
How to Respond FDA Form 483 and Warning Letters Know its differences
PolarityTE FDA Form 483
PolarityTE FDA Form 483
LOGO
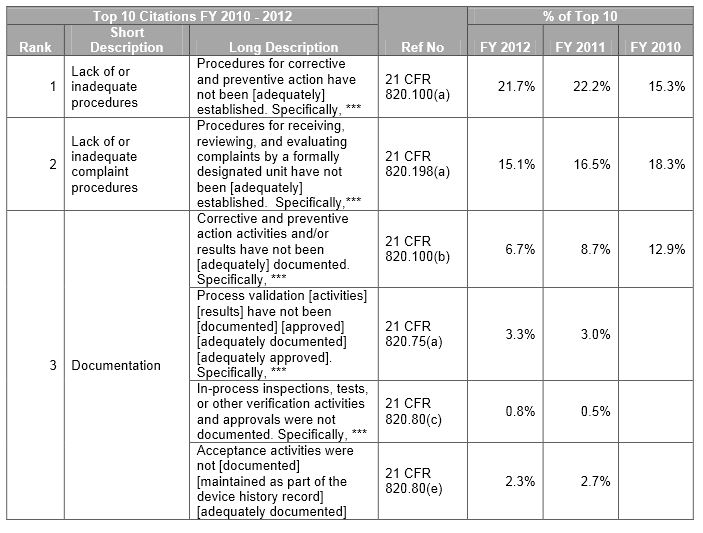
FDA Form 483 Top Ten Observations for Medical Devices SPK and Associates
Intarcia Therapeutics Form 483 Food And Drug Administration Business
LOGO
LOGO
US FDA Form 483 to Aurobindo Pharma Ltd Unit VI
Related Post: