Does Nh3 Form Hydrogen Bonds
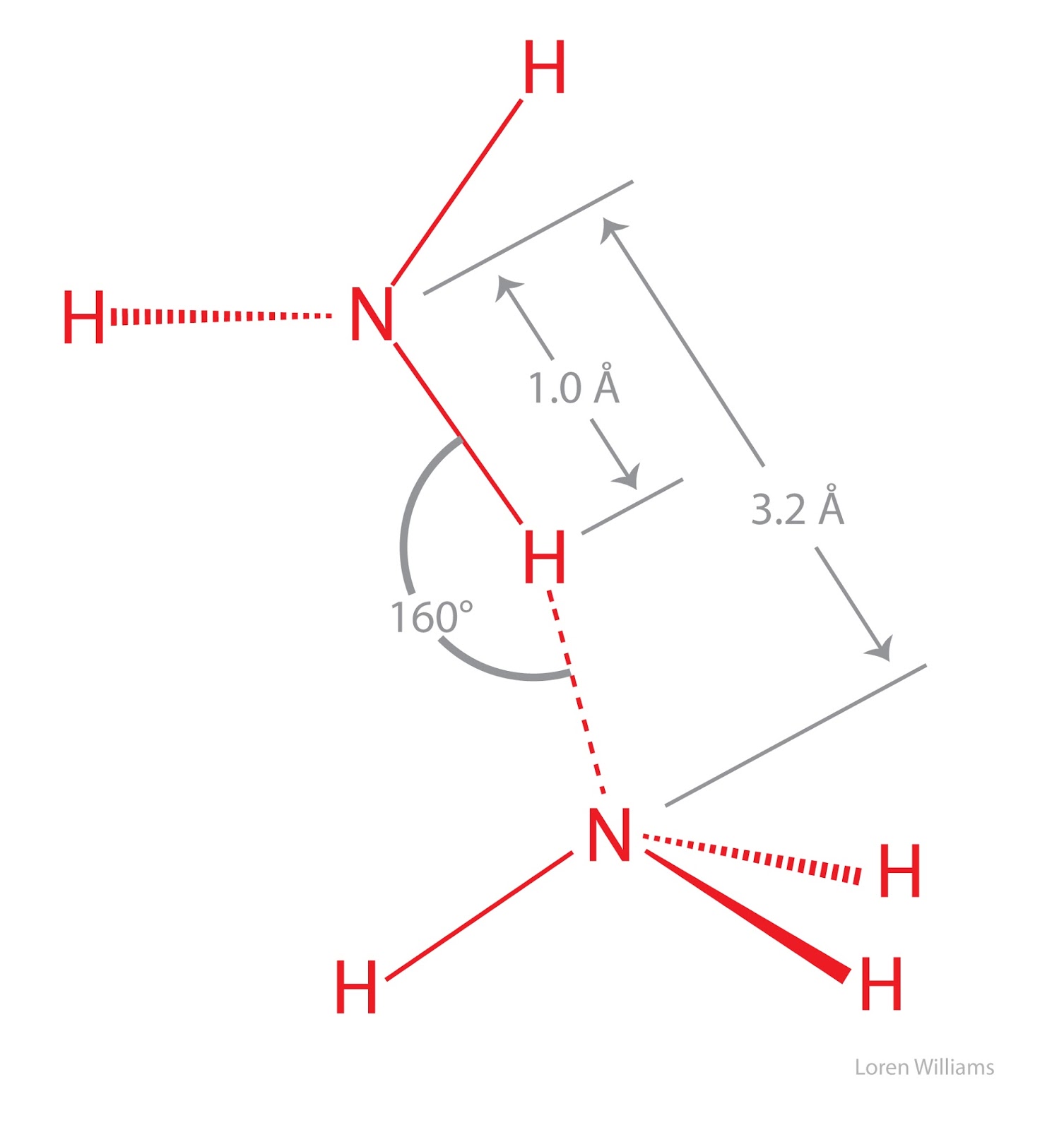
Does Nh3 Form Hydrogen Bonds - Web yes, nh3 forms hydrogen bonds. Even in interactions that do not. It is easily liquefied due to the strong hydrogen bonding between molecules. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Therefore, nh3 does have hydrogen bonding because it has a hydrogen atom bonded to a highly electronegative nitrogen atom and is capable of forming hydrogen bonds with other nitrogen or oxygen atoms. This particular hydrogen only has its nucleus transferred—its electrons remain with chlorine. It is six for one ammonia (nh3) molecule according to the octet rule. Web as expected, nh 3 is observed to be a nearly universal proton acceptor, accepting hydrogen bonds from even some of the weakest proton donors. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being oxygen and fluorine) that can form hydrogen bonds when hydrogen (h) is attached to them. Ammonia can participate in hydrogen bonding but does not have a hydrogen bond. If you liken the covalent bond between the oxygen and hydrogen to a stable marriage, the hydrogen bond has just good friends status. 5 however, the nature of hydrogen bond and its lifetime in liquid ammonia have remained an enigma. Due to the electronegativity difference between the nitrogen atom and hydrogen, a partial negative charge develops on nitrogen while a. Because the n atom in nh3 is more. Web a molecule of ammonia can give and receive up to three hydrogen bonds. At any one time only about 1% of the ammonia has actually reacted to form ammonium ions. Look for the total number of bonds forming: Web nh 3 (ammonia) does have hydrogen bonding. Ammonia is a colourless gas with a characteristically pungent smell. Hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Surprisingly, no evidence has been found to support the view that nh 3 acts as. Web in nh3, the hydrogen atom is attracted to the lone pair of. The reversible arrows show that the reaction doesn't go to completion. So yes, we can have hydrogen bonding between one h2o molecule and one hcl molecule, in which case the o molecule in h2o forms a hydrogen bond with the h from hcl. It is six for one ammonia (nh3) molecule according to the octet rule. ( b) map illustrating. 6,7 within the concept of associated liquids, which are characterized by a. Hence, nh 3 can form hydrogen bonds. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. 5 however, the nature of hydrogen bond and its lifetime in liquid ammonia have remained an enigma. The reversible arrows show that the reaction doesn't go to completion. Web in nh3, the hydrogen atom is attracted to the lone pair of electrons on another nitrogen atom or an oxygen atom in a neighboring molecule. N has small atomic size and high electronegativity. 6,7 within the concept of associated liquids, which are characterized by a. It is six for one ammonia (nh3) molecule according to the octet rule. Web. Hence, nh 3 can form hydrogen bonds. If you liken the covalent bond between the oxygen and hydrogen to a stable marriage, the hydrogen bond has just good friends status. Web nh 3 + group additionally forms hydrogen bonds to two other carbonyl sites and crystallographic water (shown by dashed lines). Hydrogen bonds can form between different molecules, as long. Web surprisingly, no evidence has been found to support the view that nhx3 n h x 3 acts as a proton donor through hydrogen bonding, ( b) map illustrating relative positioning of the lysine nh 3. Web hydrogen bonds have about a tenth of the strength of an average covalent bond, and are being constantly broken and reformed in liquid. It is lighter than air, its density being 0.589 times that of air. Web nh 3 + group additionally forms hydrogen bonds to two other carbonyl sites and crystallographic water (shown by dashed lines). So, the bond between this particular hydrogen atom and the central nitrogen is a dative covalent bond. Ammonia is a colourless gas with a characteristically pungent. At any one time only about 1% of the ammonia has actually reacted to form ammonium ions. So yes, we can have hydrogen bonding between one h2o molecule and one hcl molecule, in which case the o molecule in h2o forms a hydrogen bond with the h from hcl. Web yes, nh3 forms hydrogen bonds. Web the concept of hydrogen. If you liken the covalent bond between the oxygen and hydrogen to a stable marriage, the hydrogen bond has just good friends status. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. 5 however, the nature of hydrogen bond and its lifetime in liquid ammonia have remained an enigma. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Chemprime (moore et al.) 8: Web n h 3 + h cl → n h 4cl. The reversible arrows show that the reaction doesn't go to completion. Web in nh3, the hydrogen atom is attracted to the lone pair of electrons on another nitrogen atom or an oxygen atom in a neighboring molecule. Hence, ph 3 cannot form hydrogen bonds. 6,7 within the concept of associated liquids, which are characterized by a. The solubility of ammonia is mainly due to the hydrogen bonding and not the reaction. Ammonia molecules joined together by hydrogen bonds makeup ammonia clusters. It is easily liquefied due to the strong hydrogen bonding between molecules. Web to understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. 1 nitrogen atom needs 3 electrons and all 3 hydrogen atoms need 1 more electron to get stable. P has large size and low electronegativity. Ammonia is a colourless gas with a characteristically pungent smell. Because the n atom in nh3 is more. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being oxygen and fluorine) that can form hydrogen bonds when hydrogen (h) is attached to them. Web a molecule of ammonia can give and receive up to three hydrogen bonds.Does NH3 have Hydrogen Bonding Techiescientist
PPT Ammonia (NH 3 ) PowerPoint Presentation, free download ID2053577
Hydrogen Bonding in Ammonia (NH3) YouTube
[Solved] Ammonia, NH3, exhibits hydrogen bonding. Using five molecules
How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend
chemistry Intermolecular Hydrogen Bonding
Hydrogen Bonding Definition, Example, Types, Question Embibe
savvychemist Intermolecular Forces (3) Hydrogen Bonding
hydrogen bond types Best Chemistry Blogs & Tutorials Digital
How do Hydrogen bonds form in H2O NH3 HF Hydrogen Bonding
Related Post: