Do Metals Form Positive Or Negative Ions
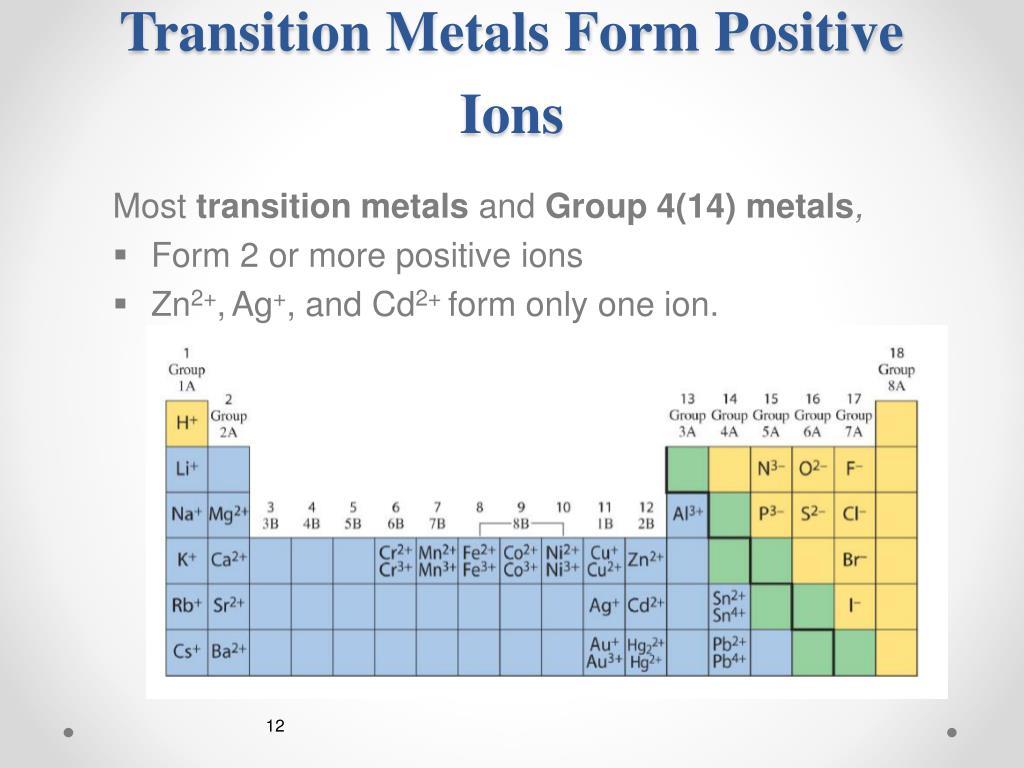
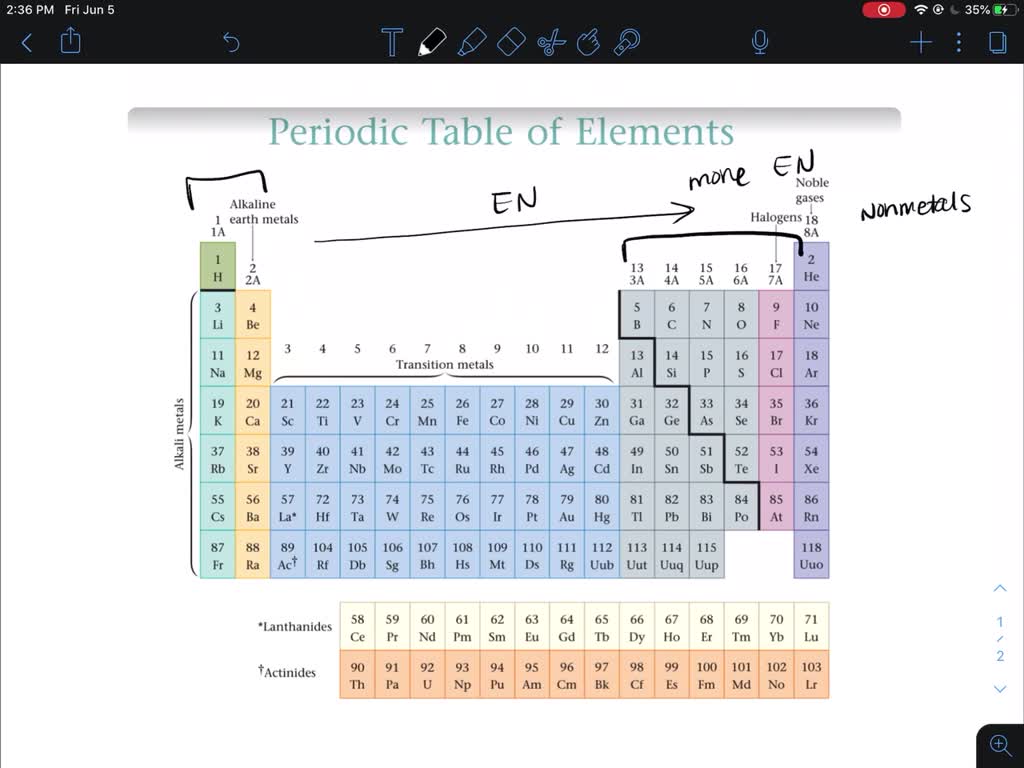
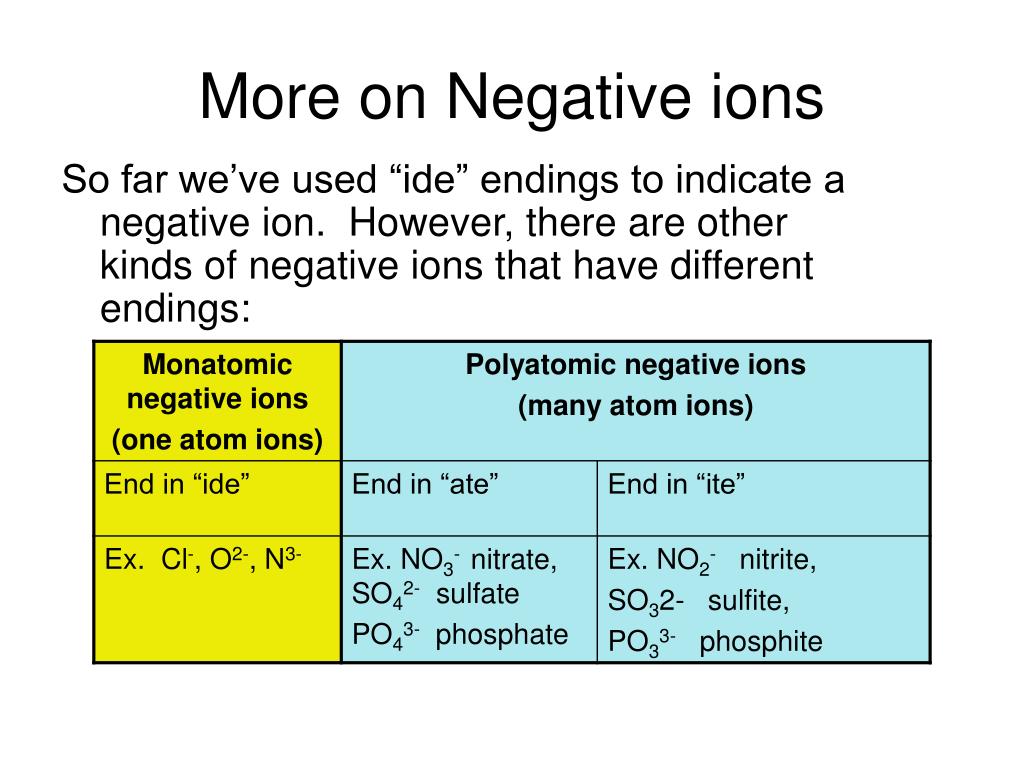
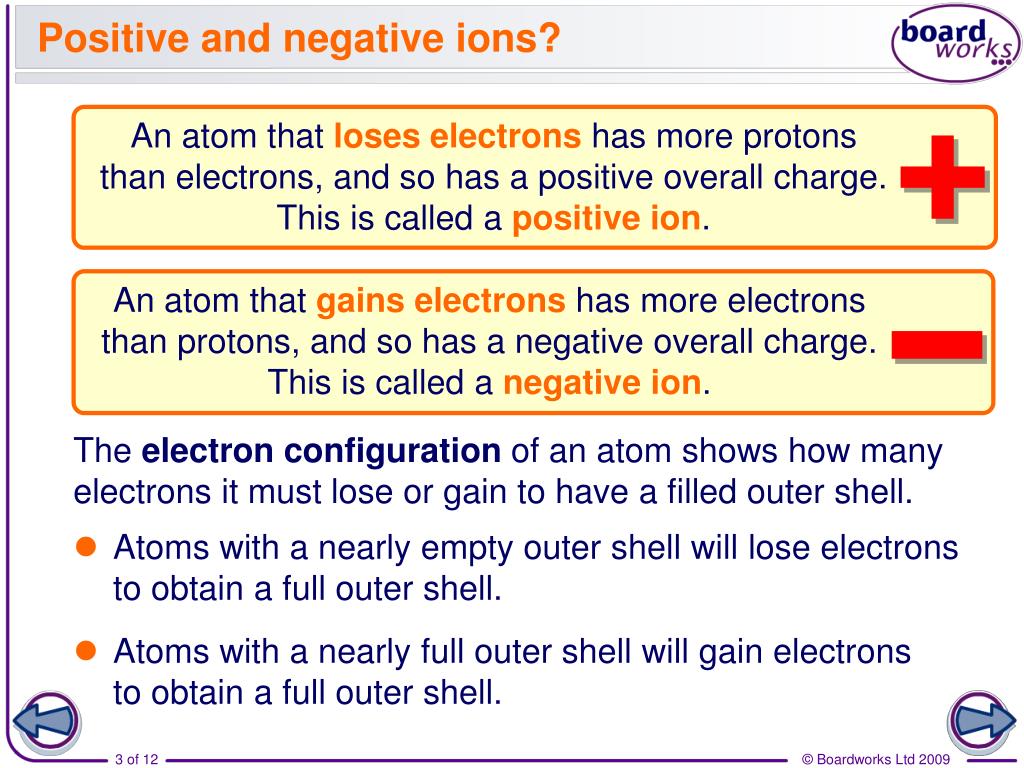
Do Metals Form Positive Or Negative Ions - Positively charged ions are called cations; Metals tend to form cations, while nonmetals tend to form anions. Metal atoms lose electrons to. Web best answer copy no, metals do not form negative ions: Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Web ionic compounds generally form from metals and nonmetals. Web this is actually one of the chemical properties of metals and nonmetals: Second, most atoms form ions of a. Ions form when atoms lose or gain electrons. Web in general, the ionization energy of metals is much lower than the ionization energy of nonmetals, which is why, in general, metals will lose electrons to form positively. Metals tend to have low ionization energies, and typically lose electrons (i.e. For example, iron (fe) atoms can form 2+ cations or 3+ cations. Web ionic compounds generally form from metals and nonmetals. Metals tend to form cations, while nonmetals tend to form anions. Alkali metals can form negative ions as explained in this answer, and halogens can also form. Positively charged ions are called cations; A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. And thus metals tend to form positive ions. Web in general, the ionization energy of metals is much lower than the ionization energy of nonmetals, which is why, in general, metals will. Web ionic compounds generally form from metals and nonmetals. Web metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web metals form positive ions (cations). Cobalt (co) is another element that can form. Positively charged ions are called cations; Metals tend to have low ionization energies, and typically lose electrons (i.e. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web metals usually form positive ions because metals are very “electropositive”. M + δ → m 2+ + 2e− at. Web of course, exceptions exist in most cases. Ionic formulas balance the total positive and. Metal atoms lose electrons to. Web metals usually form positive ions because metals are very “electropositive”. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Web a few elements, all metals, can form more than one possible charge. M + δ → m 2+ + 2e− at an atomic level, the valence electrons of the metal are conceived to be. Web metals form positive ions (cations). Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Web transition metals. Web best answer copy no, metals do not form negative ions: Web ionic compounds generally form from metals and nonmetals. That means that the outer electrons of each atom of a metal are very. M + δ → m 2+ + 2e− at an atomic level, the valence electrons of the metal are conceived to be. Compounds that do not. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. Web metals usually form positive ions because metals are very “electropositive”. Metal atoms lose electrons to. For. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. Web metals form positive ions (cations). For example, iron (fe) atoms can form 2+ cations or 3+ cations. Cobalt (co) is. Web best answer copy no, metals do not form negative ions: M + δ → m 2+ + 2e− at an atomic level, the valence electrons of the metal are conceived to be. Cobalt (co) is another element that can form. Ionic formulas balance the total positive and. Web ionic compounds generally form from metals and nonmetals. Web metals form positive ions (cations). Web ionic compounds generally form from metals and nonmetals. Web ion is an atom or group of atoms with a positive or negative charge. Web metals usually form positive ions because metals are very “electropositive”. Ionic formulas balance the total positive and. Metals tend to form cations, while nonmetals tend to form anions. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. Alkali metals can form negative ions as explained in this answer, and halogens can also form positive atomic ions. Web best answer copy no, metals do not form negative ions: Web this is actually one of the chemical properties of metals and nonmetals: Many transition metals can form more than one ion. That means that the outer electrons of each atom of a metal are very. Ions form primarily through loss of \(s\) electrons. Metals tend to have low ionization energies, and typically lose electrons (i.e. M + δ → m 2+ + 2e− at an atomic level, the valence electrons of the metal are conceived to be. And thus metals tend to form positive ions. Cobalt (co) is another element that can form. Second, most atoms form ions of a. Web transition metals have unfilled inner \(d\) electron shells. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon.PPT How do atoms form ions? PowerPoint Presentation, free download
Do Metals Form Positive Or Negative Ions Printable Form, Templates
Chem matters ch6_ionic_bond
Chem matters ch6_ionic_bond
Bohr Diagrams of Ions Presentation Chemistry
PPT Naming Ionic Compounds PowerPoint Presentation, free download
Do Metals Form Positive Or Negative Ions Printable Form, Templates
Ions
PPT Naming Ionic Compounds PowerPoint Presentation, free download
PPT How do atoms form ions? PowerPoint Presentation, free download
Related Post:


.PNG)