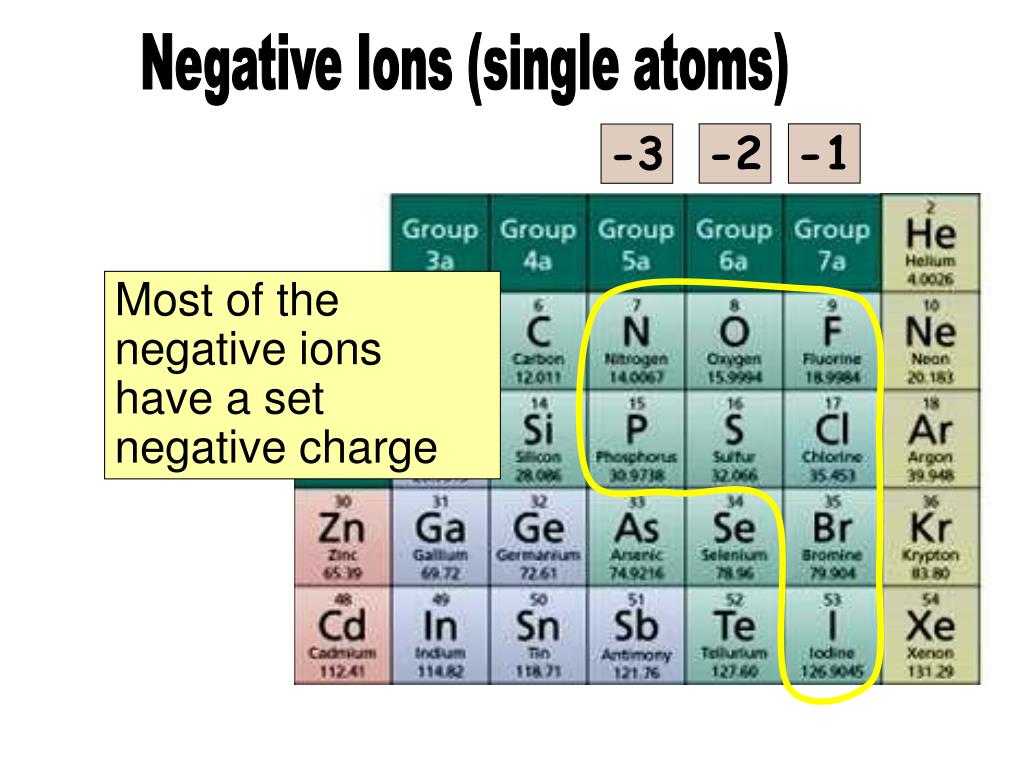
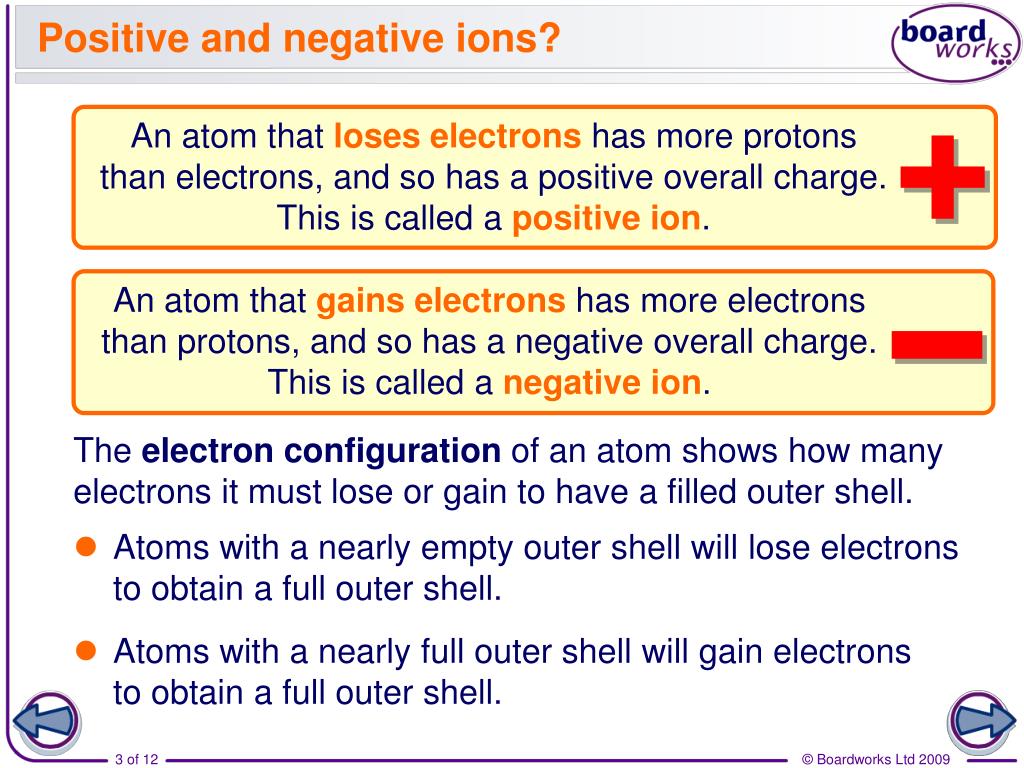
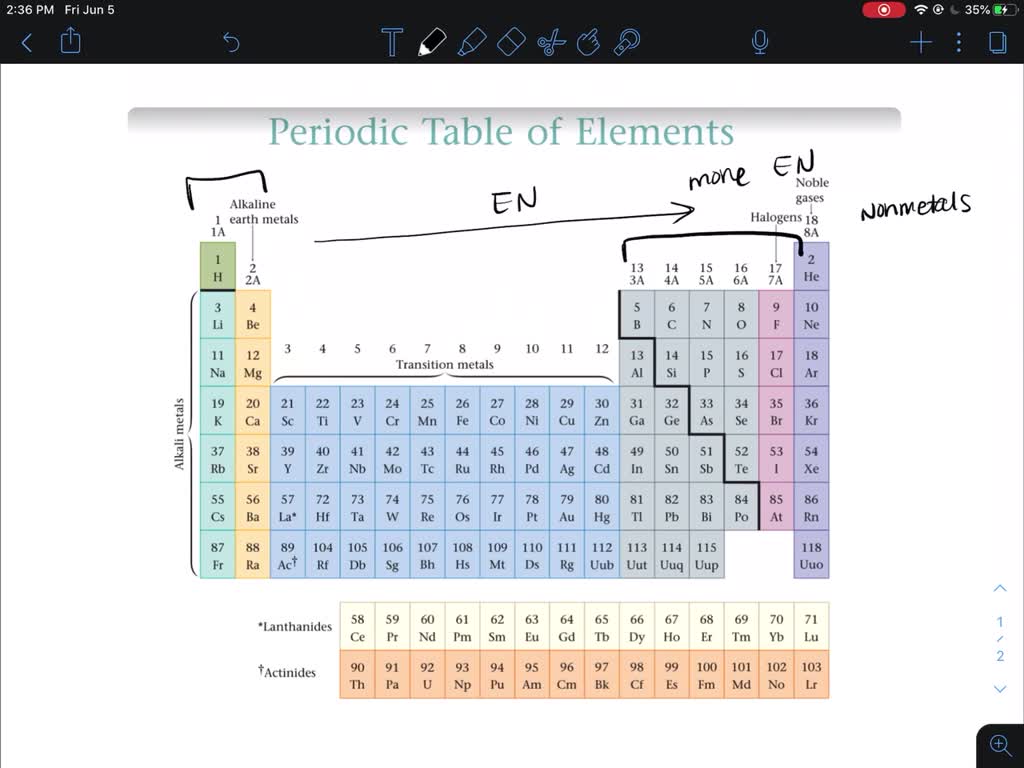
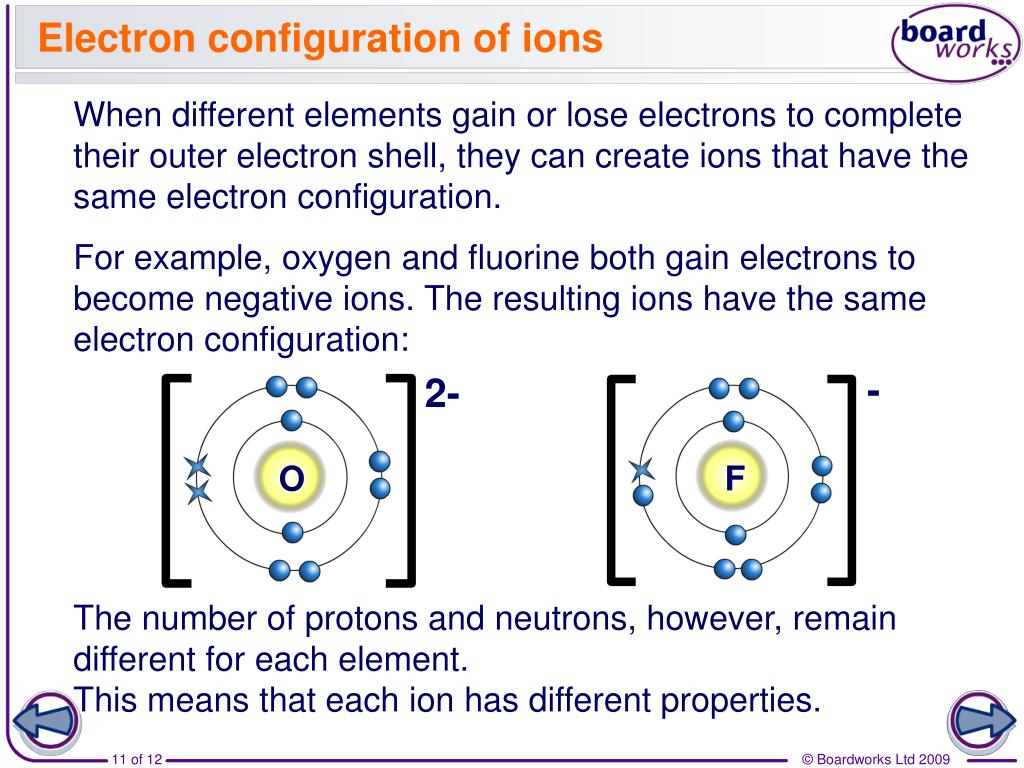
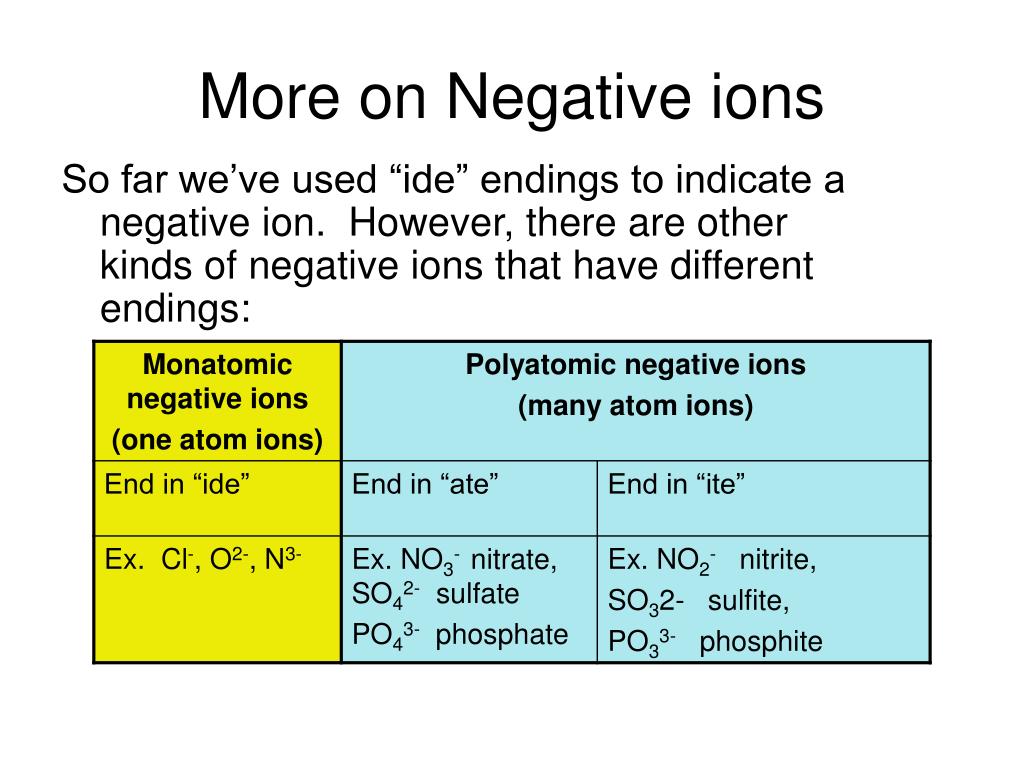
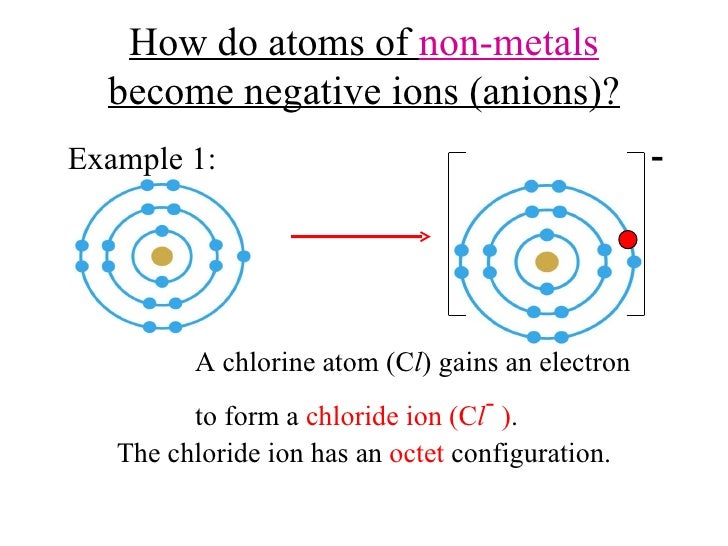
Do Metals Form Negative Ions
Do Metals Form Negative Ions - Ionic formulas balance the total positive and negative charges. At an atomic level, the valence electrons of the metal are conceived to be delocalized. Thus, the electron affinity will be. Web the answer is usually a metal. At least at first glance, it's not an element that suggests metallic properties. Web metal atoms lose electrons to form positive ions ( cations. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Web ions form when atoms lose or gain electrons. Web because they show no tendency to form negative ions, the kind of bonding present in ionic solids can immediately be ruled out. Web we would like to show you a description here but the site won’t allow us. Ionic compounds have positive ions and negative ions. The ions are positive, because. A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas, neon. Metal atoms lose electrons from their outer shell when they form ions: Explore book buy on amazon. Web most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. Why do metal atoms form ions? Positively charged ions are called cations, and negatively charged ions are called anions. Web thus, nonmetals tend to form negative ions. Explore book buy on. A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas, neon. Web atoms gain electrons to form negatively charged ions. Thus, the electron affinity will be. Why do metal atoms form ions? When writing the charge on the ion, remember to put the number before the positive or. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Web thus, nonmetals tend to form negative ions. Web the answer is usually a metal. The ions are positive, because. Then, there's hydrogen, a colorless and odorless gas. And thus metals tend to form positive ions. M + δ → m 2+ + 2e−. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. In this ocr gcse chemistry study guide, we'll go through the group 0 elements, the noble. Web ions form when atoms lose or gain electrons. In this ocr gcse chemistry study guide, we'll go through the group 0 elements, the noble gases, are all unreactive. Web nonmetals form negative ions (anions). Metal atoms lose electrons from their outer shell when they form ions: When writing the charge on the ion, remember to put the number before. Web we would like to show you a description here but the site won’t allow us. Web because they show no tendency to form negative ions, the kind of bonding present in ionic solids can immediately be ruled out. Web the answer is usually a metal. Web nonmetals form negative ions (anions). Web most transition metals differ from the metals. Web thus, nonmetals tend to form negative ions. Then, there's hydrogen, a colorless and odorless gas. The mobility of the electron fluid. At an atomic level, the valence electrons of the metal are conceived to be delocalized. Why do metal atoms form ions? Web metal atoms lose electrons to form positive ions ( cations. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. When writing the charge on the ion, remember to put the number before the positive or negative symbol (2+). Web because. Web negative ions are called anions close anion an atom or group of atoms that have gained electrons and become negatively charged. The mobility of the electron fluid. Web the answer is usually a metal. At an atomic level, the valence electrons of the metal are conceived to be delocalized. Web ions form when atoms lose or gain electrons. Why do metal atoms form ions? In this ocr gcse chemistry study guide, we'll go through the group 0 elements, the noble gases, are all unreactive. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); M + δ → m 2+ + 2e−. Positively charged ions are called cations, and negatively charged ions are called anions. Web atoms gain electrons to form negatively charged ions. Web because they show no tendency to form negative ions, the kind of bonding present in ionic solids can immediately be ruled out. At an atomic level, the valence electrons of the metal are conceived to be delocalized. At least at first glance, it's not an element that suggests metallic properties. The ions are positive, because. Explore book buy on amazon. Metal atoms lose electrons from their outer shell when they form ions: Web the answer is usually a metal. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Web we would like to show you a description here but the site won’t allow us. Ionic formulas balance the total positive and negative charges. When writing the charge on the ion, remember to put the number before the positive or negative symbol (2+). The mobility of the electron fluid. Web most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. Thus, the electron affinity will be.PPT How do atoms form ions? PowerPoint Presentation, free download
PPT NOMENCLATURE PowerPoint Presentation, free download ID1275392
PPT How do atoms form ions? PowerPoint Presentation, free download
Do Metals Form Positive Or Negative Ions Printable Form, Templates
Ions
Chem matters ch6_ionic_bond
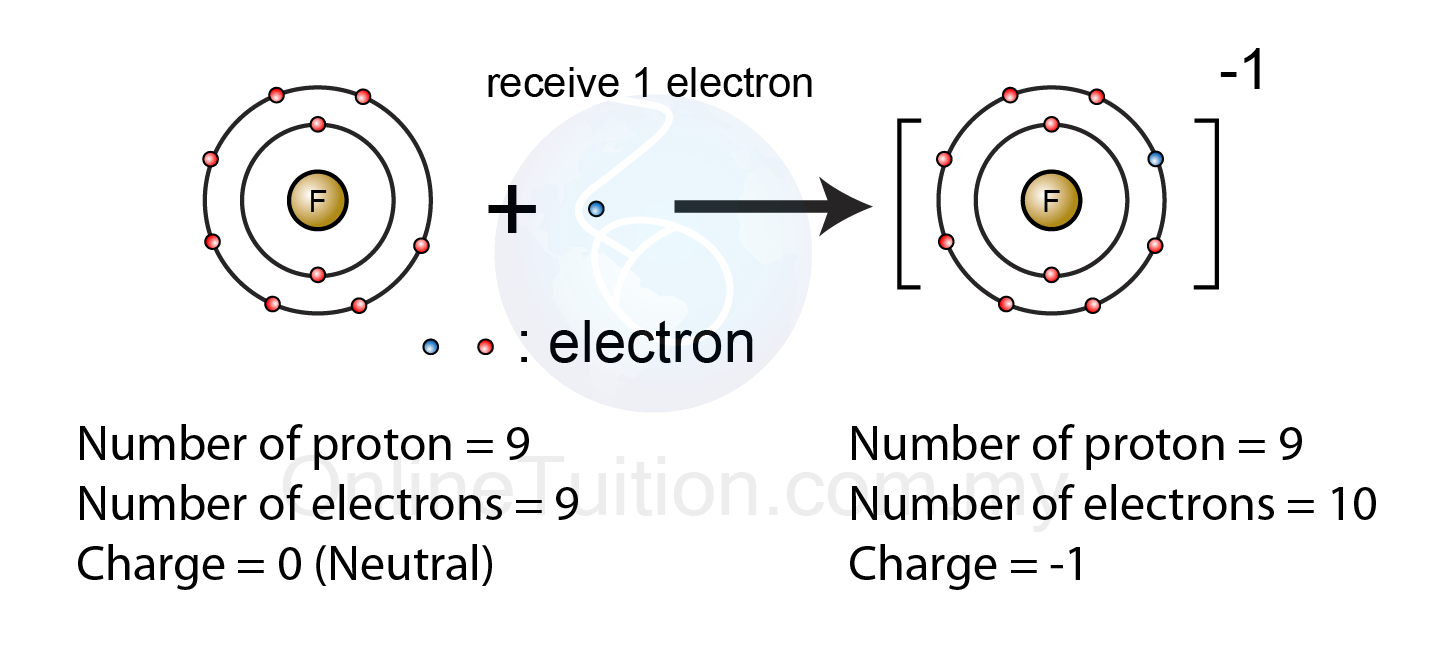
Formation of Negative Ions SPM Chemistry
PPT How do atoms form ions? PowerPoint Presentation, free download
PPT Naming Ionic Compounds PowerPoint Presentation, free download
Chem matters ch6_ionic_bond
Related Post: