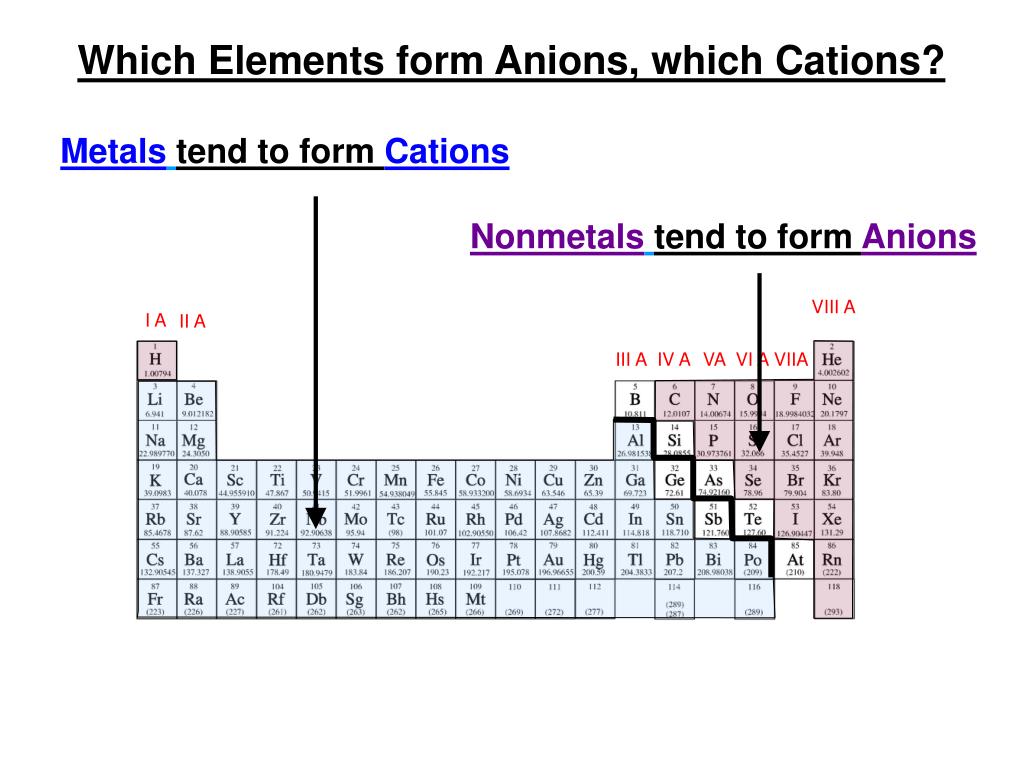
Do Metals Form Cations Or Anions
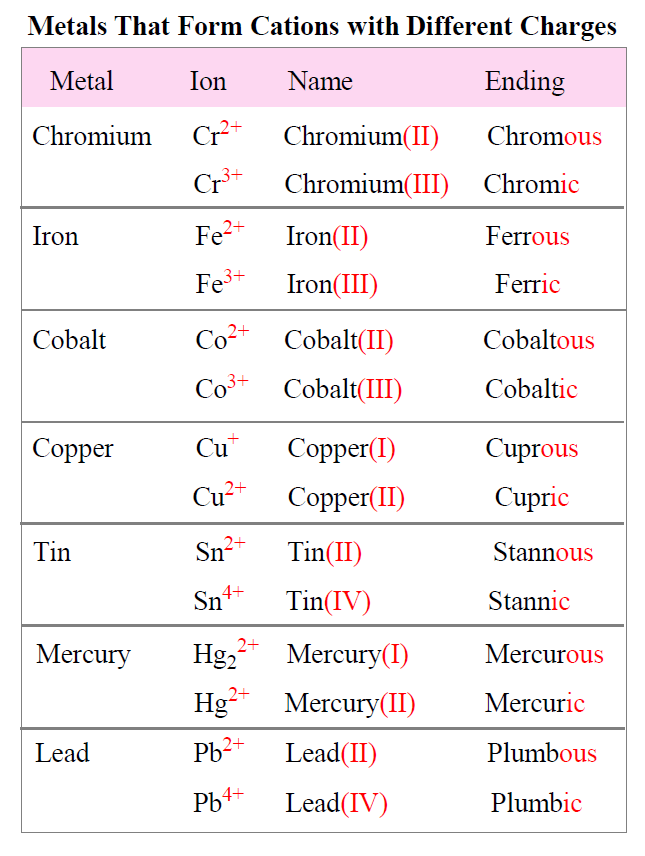
Do Metals Form Cations Or Anions - Web transition metal ions. Web metals form positive ions (cations). , while species with overall negative charges are called. Web we would like to show you a description here but the site won’t allow us. Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with. Most other metals form cations (e.g. Species with overall positive charges are termed. On the other hand, compounds formed between two or. 1 metals have low lonization energies ii. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web metals form positive ions (cations). Iron, silver, nickel), whilst most other nonmetals typically form anions (e.g. You can also tell that they form cations because some of the. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Get deals and low prices on cesium metal. Most other metals form cations (e.g. 1 metals have low lonization energies ii. Web the metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. Web we would like to show you a description here but the site won’t allow us. Any substance in elemental form is an atom or a molecule. Web transition metal ions. 1 metals have low lonization energies ii. Most other metals form cations (e.g. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble. Figure \(\pageindex{2}\) lists the ions (cations and anions) that. You can also tell that they form cations because some of the. They become ions depending upon the the electronic configuration. 1 metals have low lonization energies ii. Iron, silver, nickel), whilst most other nonmetals typically form anions (e.g. On the other hand, compounds formed between two or. 1 metals have low lonization energies ii. Web metals form positive ions (cations). Web transition metal ions. Web metals form positive ions (cations). Web transition metals often form ions wihout complete octets that's why all the stable ions are all cations. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Figure \(\pageindex{2}\) lists the ions (cations and anions) that. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Species with overall positive charges are termed. Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with. 1 metals have low lonization energies ii. You can also tell that they form cations because some of the. Web the metals form cations, the nonmetals form anions,. Web identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Species with overall positive charges are termed. Ad choose from a wide range of industrial & scientific equipment at amazon. , while species with overall negative charges are called. On the other hand, compounds formed between two. They become ions depending upon the the electronic configuration. This is actually one of the chemical properties. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. Web metals form positive ions (cations). On the other hand, compounds formed between two or. Web remember metals lose electrons to form cations and nonmetals gain electrons to form anions. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. ,. You can also tell that they form cations because some of the. Ad choose from a wide range of industrial & scientific equipment at amazon. Most other metals form cations (e.g. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web metals are not cations, metal ions are. Remember that ions are formed only when. , while species with overall negative charges are called. Any substance in elemental form is an atom or a molecule. Web metals form positive ions (cations). Web the metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. Web metals form positive ions (cations). Web remember metals lose electrons to form cations and nonmetals gain electrons to form anions. They become ions depending upon the the electronic configuration. Web transition metals often form ions wihout complete octets that's why all the stable ions are all cations. 1 metals have low lonization energies ii. Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with. Web we would like to show you a description here but the site won’t allow us. Web identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. This is actually one of the chemical properties.PPT Mastering Chemistry PowerPoint Presentation, free download ID
Strontium Forms a Cation or Anion
The Difference Between a Cation and an Anion
PPT Lecture 4. Chapter 2. Structure of the Atom (Contd.) PowerPoint
Cations and Anions List KarsyntinOconnell
PPT IONS PowerPoint Presentation, free download ID2435906
PPT 1 Name the ions formed by these elements and classify them as
Cations and Anions Definitions, Examples, and Differences
Writing Chemical Formulas For Ionic Compounds Chemistry Steps
PPT Ionic Bonding PowerPoint Presentation, free download ID5679234
Related Post:
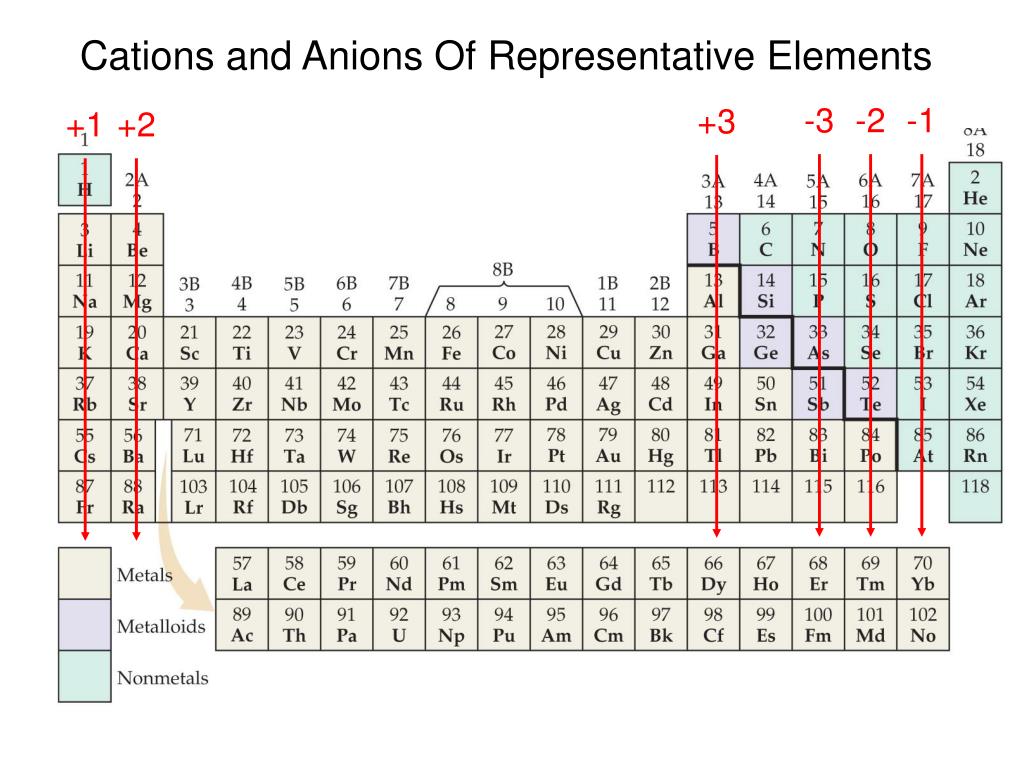

/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)