Clozapine Rems Patient Status Form
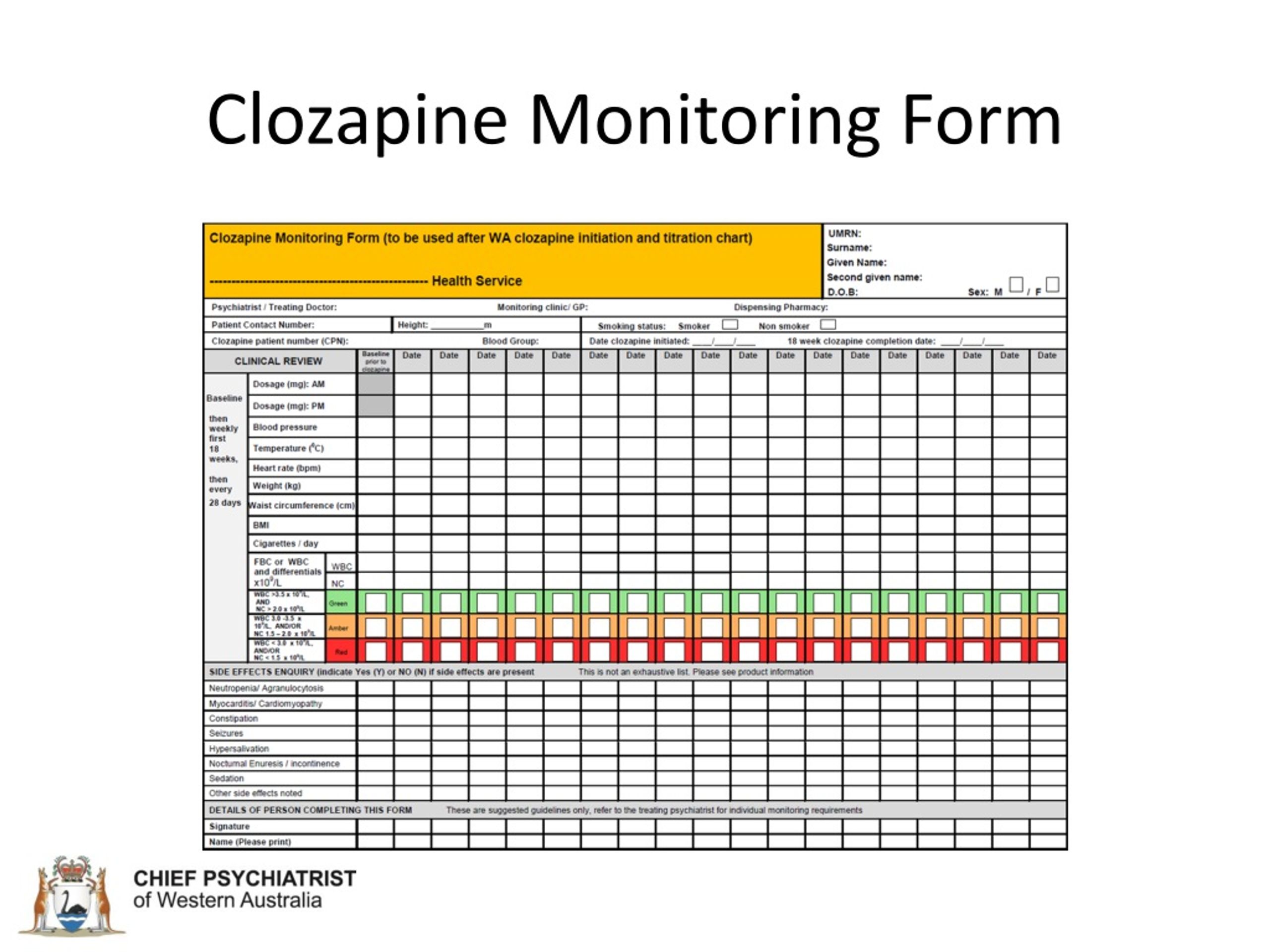
Clozapine Rems Patient Status Form - Web delaying patients getting clozapine is going to limit the extent of response. Web we encourage pharmacists and prescribers to continue working with the clozapine rems to complete certification and prescribers should continue to enroll. Web we encourage pharmacists and prescribers to continue working with the clozapine rems to complete certification and prescribers should continue to enroll. • a new patient status form (psf) must be submitted monthly by the prescriber. The patient status form is used to document the patient’s anc results,. Outpatient pharmacies • must certify in. 07/2021 for more information or materials,. Web a new patient status form will document absolute neutrophil count (anc) monitoring for all outpatients. Enroll in the clozapine rems by completing the prescriber enrollment form and submitting it to the clozapine rems. Web complete the prescriber enrollment form. This form must be submitted monthly. Web the clozapine rems (risk evaluation and mitigation strategy) is a safety program required by the food and drug administration (fda) to manage the risk of severe neutropenia. Beginning november 15, 2021, prescribers must complete and submit the patient. Web the new patient status form is used to document monitoring of the anc. Web. Web complete the prescriber enrollment form. 07/2021 for more information or materials,. Web a new patient status form will document absolute neutrophil count (anc) monitoring for all outpatients. Web dispenses clozapine to a patient, you must obtain authorization from the new clozapine rems in the form of a rems dispense authorization (rda). • a new patient status form (psf) must. 07/2021 for more information or materials,. Web a new patient status form will document absolute neutrophil count (anc) monitoring for all outpatients. Web delaying patients getting clozapine is going to limit the extent of response. Ad goodrx.com has been visited by 100k+ users in the past month Enroll in the clozapine rems by completing the prescriber enrollment form and submitting. Web we encourage pharmacists and prescribers to continue working with the clozapine rems to complete certification and prescribers should continue to enroll. Web this form will need to provide the dates and values of the most recent anc and wbc counts. Web several of these changes affect health professionals who prescribe clozapine. Web we encourage pharmacists and prescribers to continue. 1 clozapine is a second. Web dispenses clozapine to a patient, you must obtain authorization from the new clozapine rems in the form of a rems dispense authorization (rda). The form is used to document the monitoring frequency, anc values, adverse events related to. Web a new patient status form will document absolute neutrophil count (anc) monitoring for all outpatients.. Web complete the prescriber enrollment form. Web the clozapine rems (risk evaluation and mitigation strategy) is a safety program required by the food and drug administration (fda) to manage the risk of severe neutropenia. Web • must verify and document each clozapine patient’s ancs to the clozapine rems monthly, by submitting the patient status form. Web the contact center or. Web the new patient status form is used to document monitoring of the anc. Web after a rigorous screening process, 75 publications were included in the review, which focused on three main aspects: Web if a patient status form is not received, the pharmacist may use a dispense rationale to dispense (see dispense rationale below). Web a new patient status. The patient status form is used to document the patient’s anc results,. Once the override is approved, the provider will be allowed to. This form must be submitted monthly. Web this form will need to provide the dates and values of the most recent anc and wbc counts. Web a new patient status form will document absolute neutrophil count (anc). Web delaying patients getting clozapine is going to limit the extent of response. Web complete the prescriber enrollment form. Report anc via the website or anc lab reporting form. Anc reporting frequency is dependent on patient’s monitoring frequency (e.g.,. Web instead anc results will be submitted monthly through a new patient status form. Once the override is approved, the provider will be allowed to. Web delaying patients getting clozapine is going to limit the extent of response. Web we encourage pharmacists and prescribers to continue working with the clozapine rems to complete certification and prescribers should continue to enroll. Web if a patient status form is not received, the pharmacist may use a. This form must be submitted monthly. This form must be submitted monthly. Web if a patient status form is not received, the pharmacist may use a dispense rationale to dispense (see dispense rationale below). Outpatient pharmacies • must certify in. Web dispenses clozapine to a patient, you must obtain authorization from the new clozapine rems in the form of a rems dispense authorization (rda). The form is used to document the monitoring frequency, anc values, adverse events related to. Web complete the prescriber enrollment form. Web the contact center or online via the rems website. Web establish processes and procedures to verify an available, current anc is within the acceptable range for patients enrolled but not authorized to receive the drug. Web delaying patients getting clozapine is going to limit the extent of response. Web we encourage pharmacists and prescribers to continue working with the clozapine rems to complete certification and prescribers should continue to enroll. 07/2021 for more information or materials,. The patient status form is used to document the patient’s anc results,. Web the clozapine rems (risk evaluation and mitigation strategy) is a safety program required by the food and drug administration (fda) to manage the risk of severe neutropenia. Web a new patient status form will document absolute neutrophil count (anc) monitoring for all outpatients. Beginning on november 15, the clozapine rems requires a new patient status form. Once the override is approved, the provider will be allowed to. Web to add a patient status form, click the add psf button on the appropriate patient’s row. Web several of these changes affect health professionals who prescribe clozapine. Web after a rigorous screening process, 75 publications were included in the review, which focused on three main aspects:Clozapine 100 Mg Tablet, Vega Biotec Pvt Ltd, 10x10, ID 23516355497
How clozapine patients can be monitored safely and effectively The
Clozapine Tablets IP 50 MG., Prescription, Rs 53.50 /stripe Dellwich
CLOZAPINE SANDOZ COMP 30 X 100 MG Apotheek ClaeysDecraene
Spravato Rems Patient Enrollment Form Enrollment Form
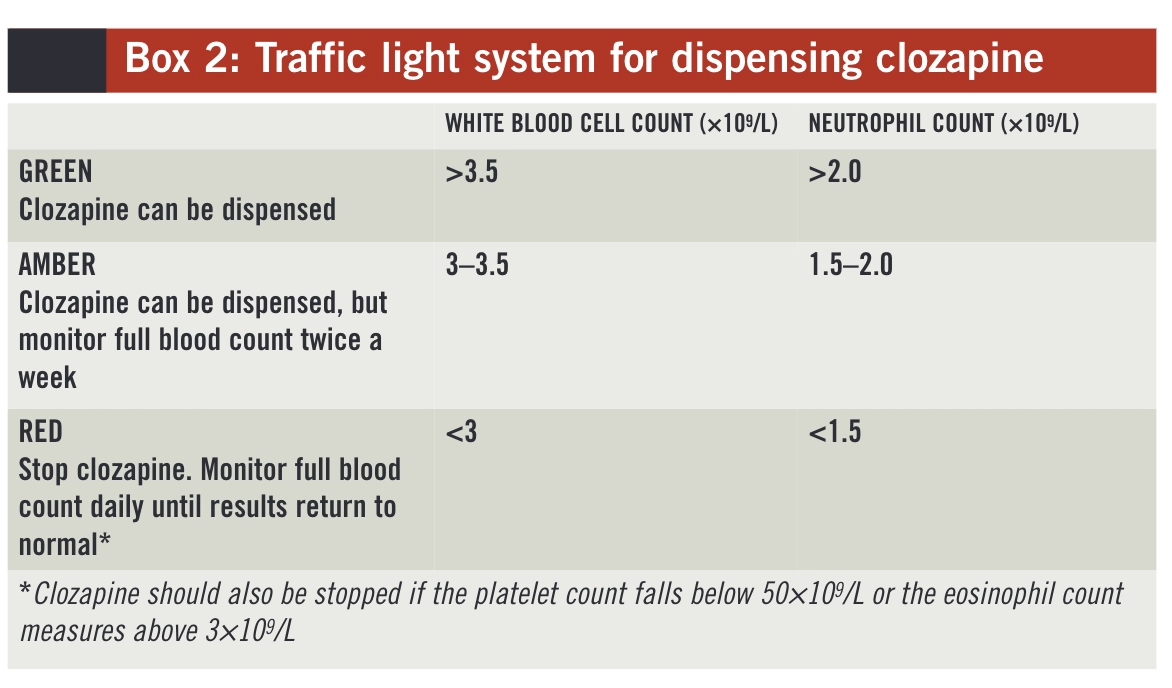
Clozapine blood monitoring for side effects of agranulocytosis
CLOZAPINE 100 MG 20 TAB price from in Egypt Yaoota!
Medication card Clozapine ACTIVE LEARNING TEMPLATES
Clozapine Prescriptiongiant
PPT Antipsychotics and Physical Healththe Clozapine Resources
Related Post: