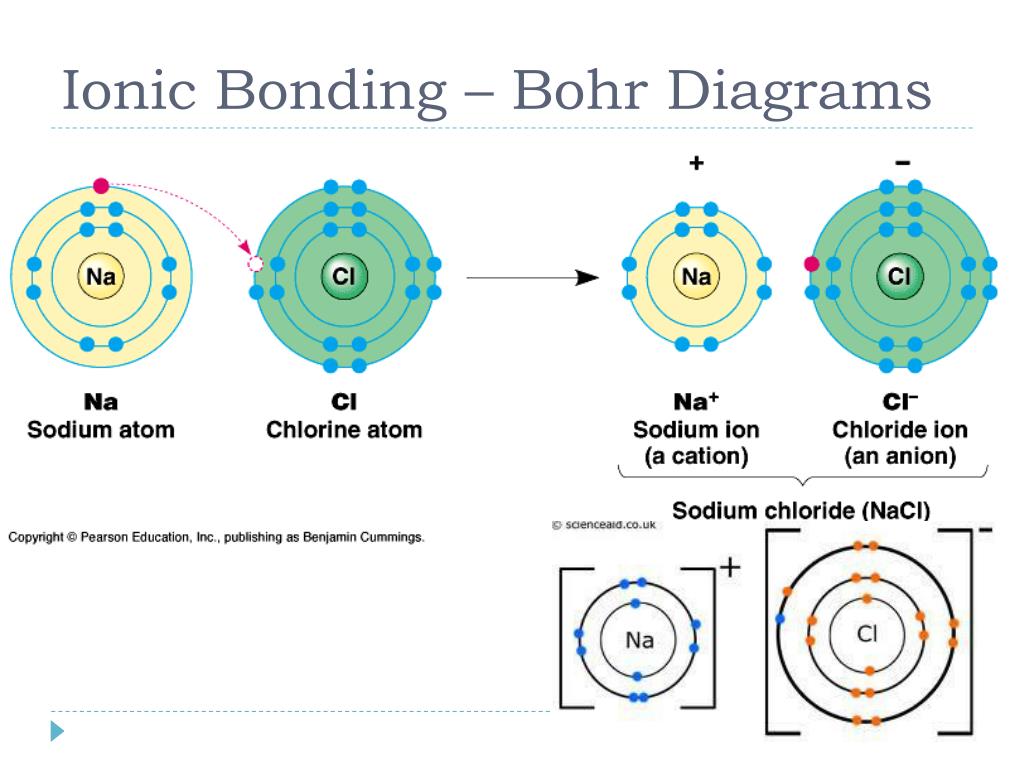
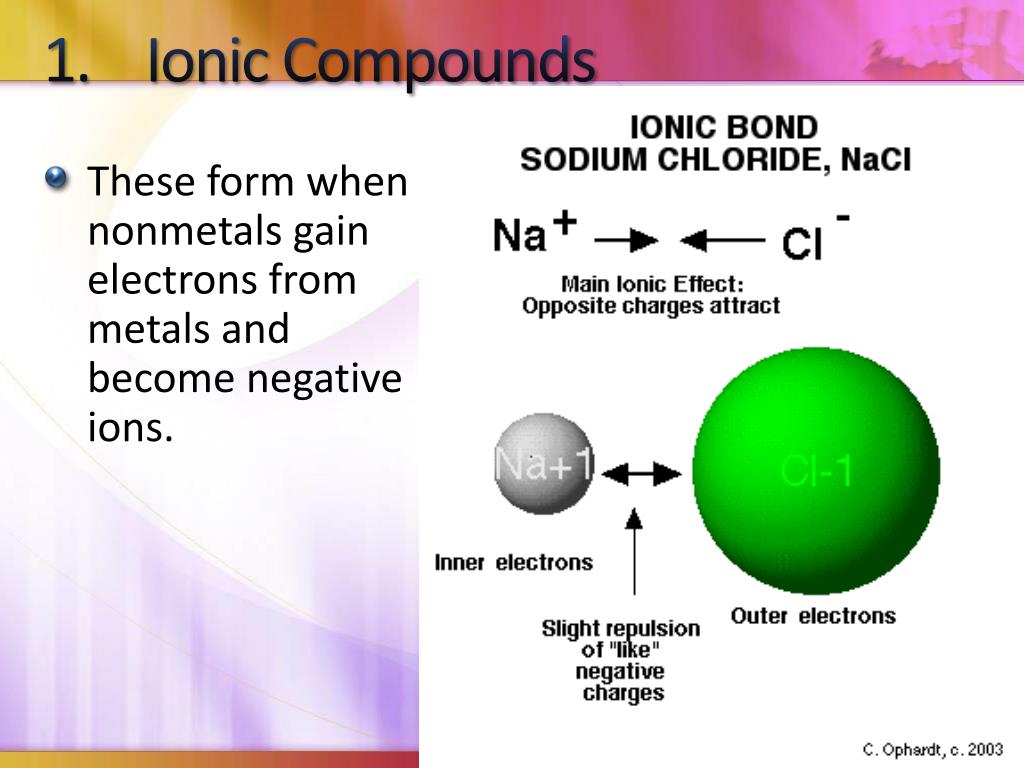
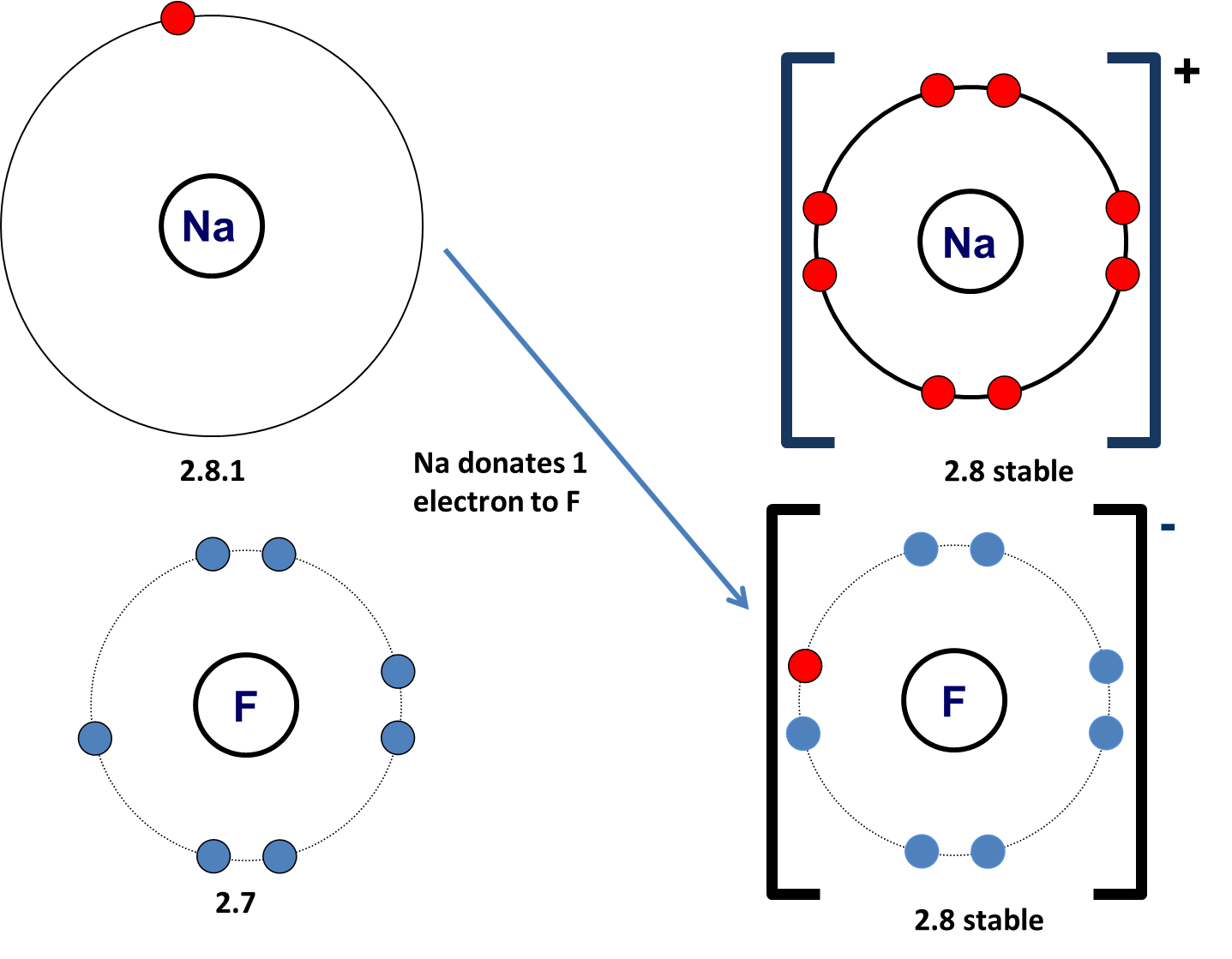
Can Two Nonmetals Form An Ionic Bond
Can Two Nonmetals Form An Ionic Bond - Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Two metals can't form an. While the difference in electronegativity between p and f (1.79) is large enough for ionic compounds to form,. The periodic table can help us recognize many of the compounds. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces of attraction between. The periodic table can help us recognize many of the compounds. (unless you consider binary acids). For now, you simply need to be able to determine. Web ∙ 11y ago. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell. Single and multiple covalent bonds. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Predicting bond type (metals vs. Web compounds composed of ions are. This process will be covered in detail in a later lecture. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Neither atom is strong enough to attract electrons from the other. The measure of this atraction ability. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is. Web i know metals and non metals make a ionic bond, and two non metals make a covalent bond, so i was just curious. Two metals can't form an. Predicting bond type (metals vs. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic bonds are created when there is big. (unless you consider binary acids). The measure of this atraction ability. Web covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. It takes two electrons to make a covalent bond, one from each bonding. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. It takes two electrons to make a covalent bond, one. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces of attraction between. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Web ∙ 11y ago. The periodic table can help us recognize many of the compounds. Two metals can't form an. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Web can metals only form ionic bonds with nonmetals or. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. For now, you simply need to be able to determine. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Web. Web the same number of electrons are accepted by the appropriate number of atoms of a nonmetal to form an octet in the anion, producing an ionic compound. It takes two electrons to make a covalent bond, one from each bonding. Web i know metals and non metals make a ionic bond, and two non metals make a covalent bond,. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces of attraction between. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Neither atom is strong enough to attract electrons from the other. Single and multiple covalent bonds.. The periodic table can help us recognize many of the compounds. Two metals can't form an. This process will be covered in detail in a later lecture. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces of attraction between. It is a type of chemical bond that generates two oppositely charged ions. The measure of this atraction ability. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Single and multiple covalent bonds. The periodic table can help us recognize many of the compounds. Web can metals only form ionic bonds with nonmetals or is it possible to have two metals in an ionic bond? For now, you simply need to be able to determine. Web covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. Web ∙ 11y ago. Neither atom is strong enough to attract electrons from the other. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions.Ionic Bonding Presentation Chemistry
How Do Ions Form Ionic Bonds
What are the differences between ionic, polar covalent, nonpolar
PPT Chemical Bonding PowerPoint Presentation, free download ID1587654
Ionic Bond Definition, Types, Properties & Examples
Ionic Bond Definition, Types, Properties & Examples
Ionic Bond Definition, Types, Properties & Examples
PPT Chapter 19 Elements And Their Properties PowerPoint Presentation
Ionic bonding Wikipedia
Structure and bonding GCSE chemistry
Related Post:
.PNG)