Can Serine Form Hydrogen Bonds
Can Serine Form Hydrogen Bonds - Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. Furthermore, this group can form a hydrogen bond with. While the sidechain is electrically neutral, this. The most common bond arrangement is a four. Web backbone carbonyls form bifurcated hydrogen bonds. Polar (uncharged) serine differs from alanine in that one of the methylenic hydrogens is. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain). One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain). Furthermore, this group can form a hydrogen bond with. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Polar (uncharged) serine differs from. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Below is the structure of the amino acid, serine. Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. Answer only one, the one at the very top which is attached to the highly electrongative. One of the most useful. Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. (l1) is. Four hydrogen atoms in the compound can form hydrogen bonds. While the sidechain is electrically neutral, this. Web water as a perfect example of hydrogen bonding. (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain). Answer only one, the one at the very top which is attached to the highly electrongative. Web backbone carbonyls form bifurcated hydrogen bonds. (l1) is. While the sidechain is electrically neutral, this. The most common bond arrangement is a four. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Below is the structure of the amino acid, serine. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain). Polar (uncharged) serine differs from alanine. Answer only one, the one at the very top which is attached to the highly electrongative. Furthermore, this group can form a hydrogen bond with. Web backbone carbonyls form bifurcated hydrogen bonds. Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. Web how many hydrogens in figure \(\pageindex{1}\) can form. Web water as a perfect example of hydrogen bonding. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? While the sidechain is electrically neutral, this. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g.,. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Below is the structure of the amino acid, serine. Four hydrogen atoms in the compound can form hydrogen bonds. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of. Four hydrogen atoms in the compound can form hydrogen bonds. The most common bond arrangement is a four. (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain). Furthermore, this group can form a hydrogen bond with. Below is the structure of the amino acid, serine. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Answer only one, the one at the very top which is attached to the highly electrongative. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. Web water as a perfect example of hydrogen bonding. While the sidechain is electrically neutral, this. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Polar (uncharged) serine differs from alanine in that one of the methylenic hydrogens is. Web backbone carbonyls form bifurcated hydrogen bonds.amino acids salt bridge vs hydrogen bond Chemistry Stack Exchange
Zwitterions of Lserine in forms I (a), II (b), III (c). Hydrogen
molecular structure Why do AsnSer and GlnThr have different H
Serine substitutions in proteins Flickr Photo Sharing!
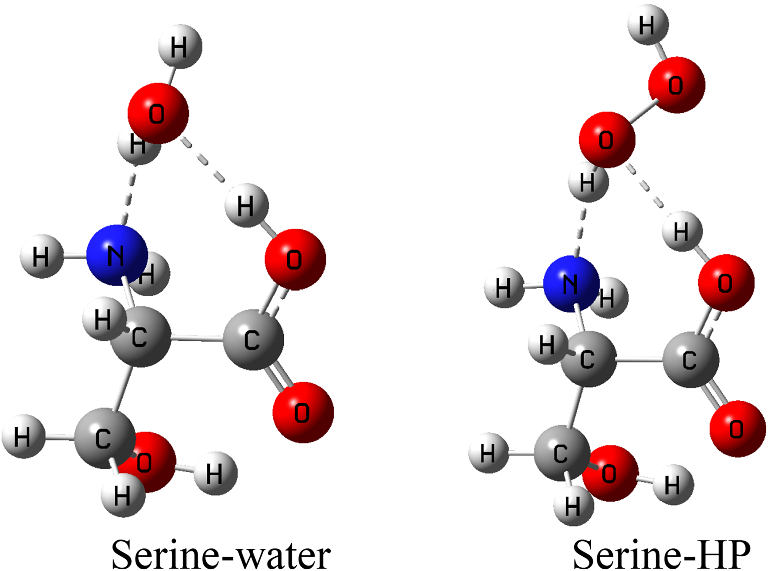
Quantum chemical study of hydrogenbonded complexes of serine with
Zwitterions of Lserine in forms I (a), II (b), III (c). Hydrogen
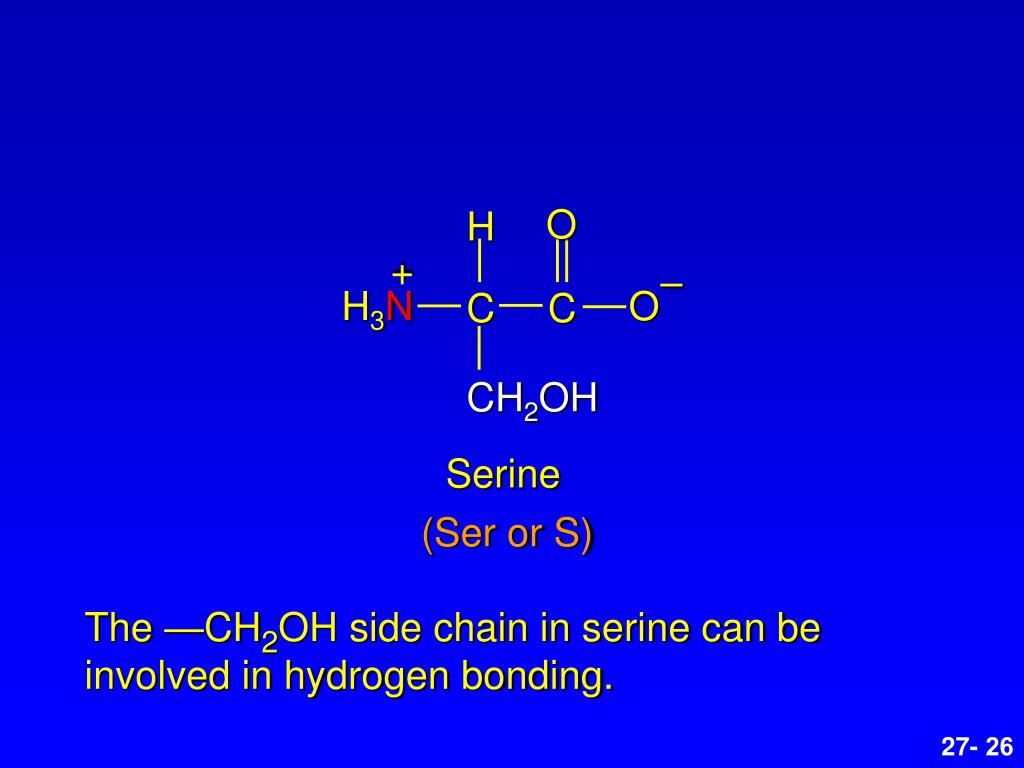
PPT Amino Acids, Peptides, and Proteins PowerPoint Presentation, free
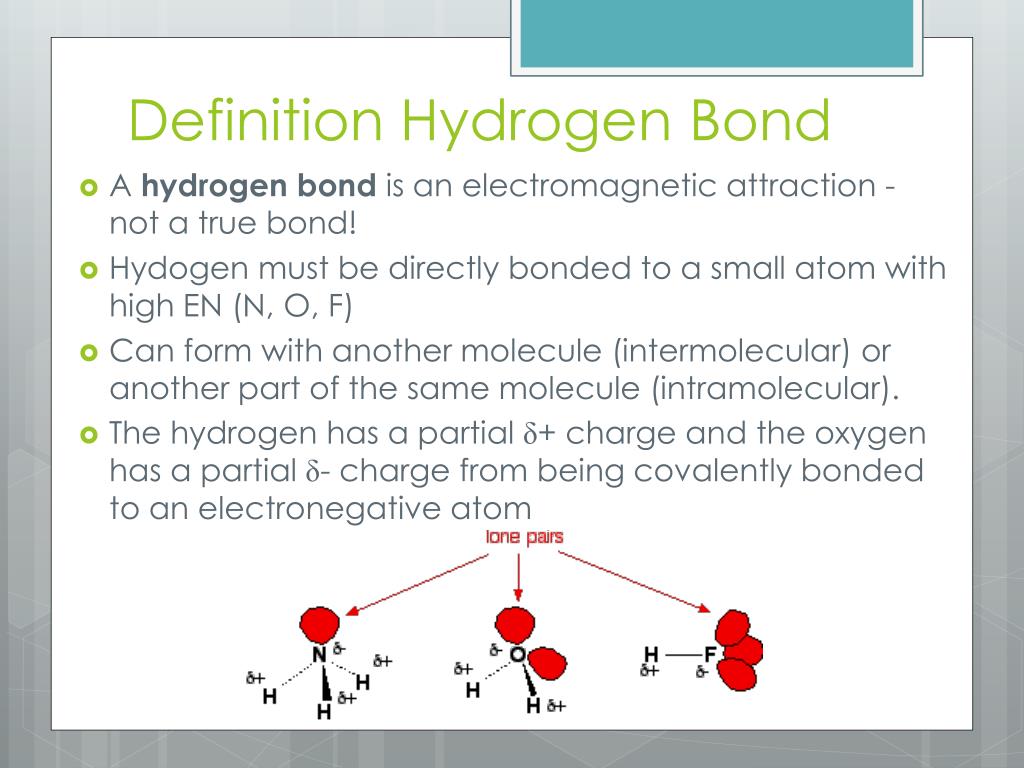
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Rules for Hydrogen bonding? Mcat
Hydrogen bond diagrams of functional nests. (a) In serine proteases
Related Post: