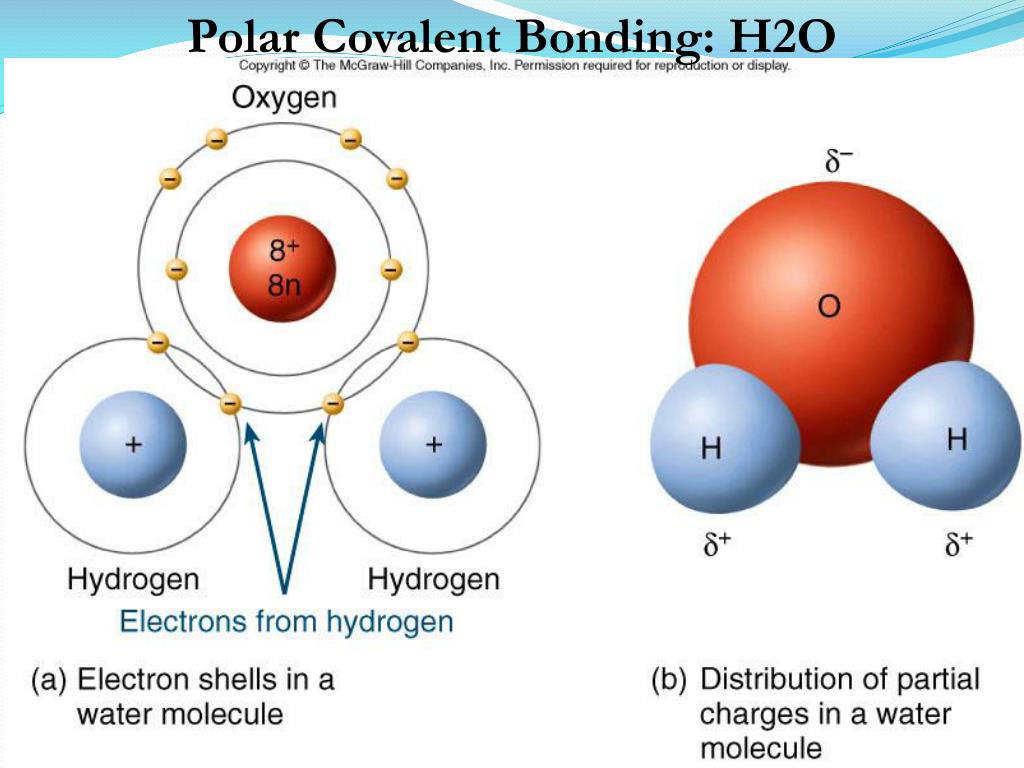
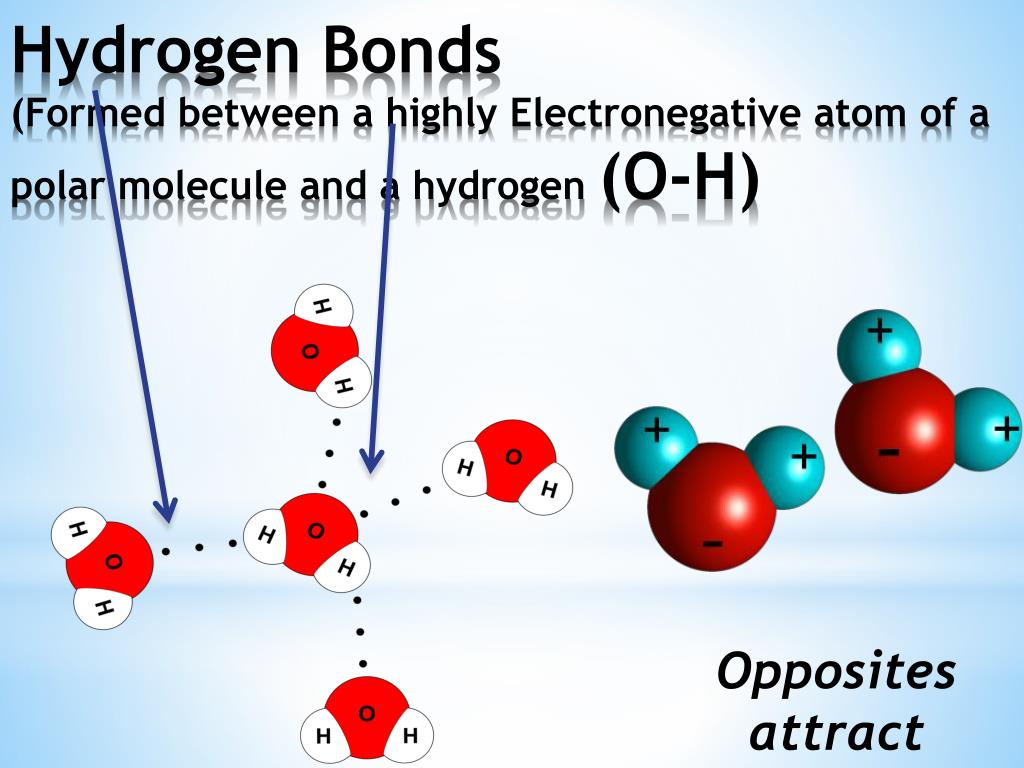
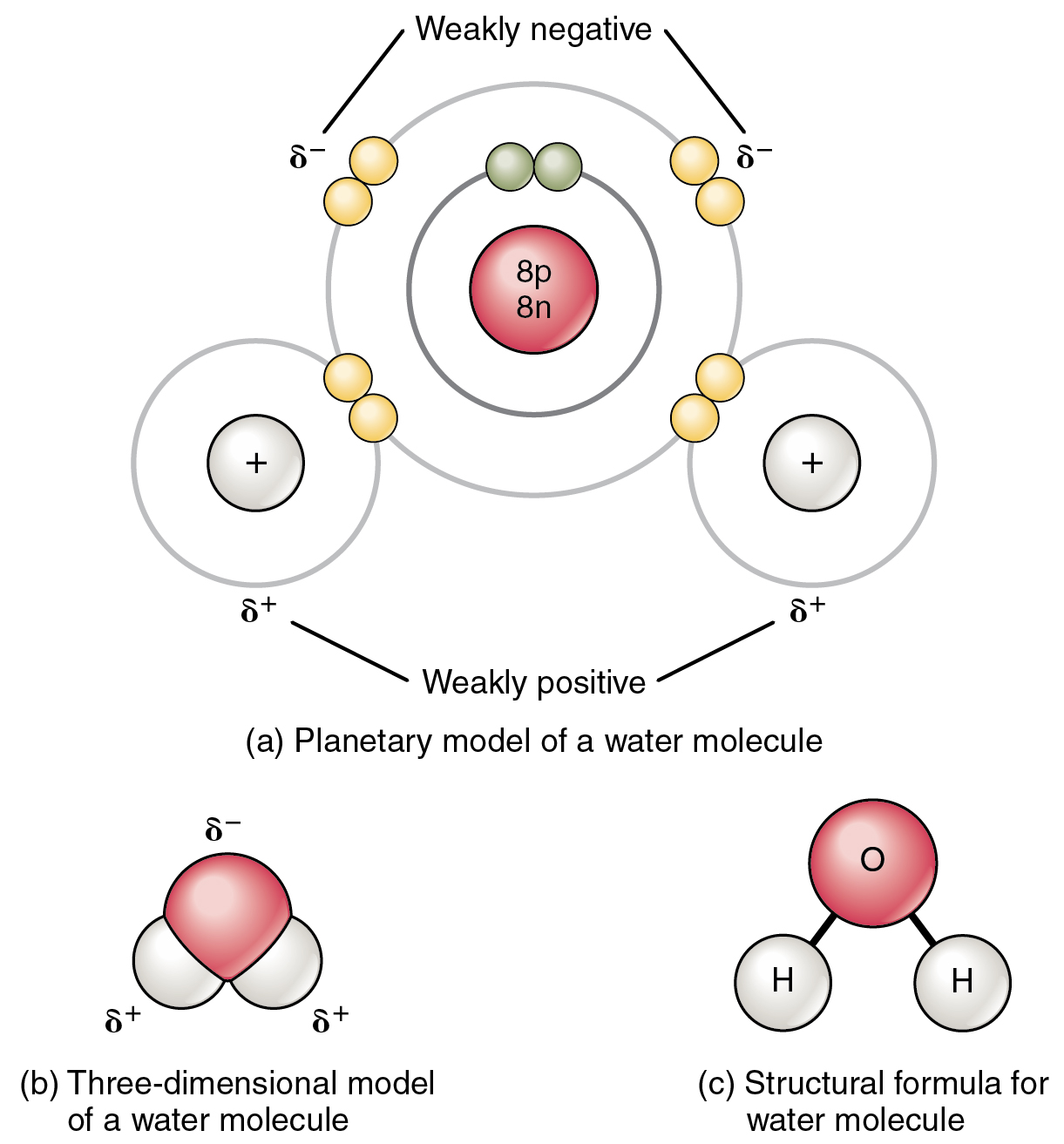
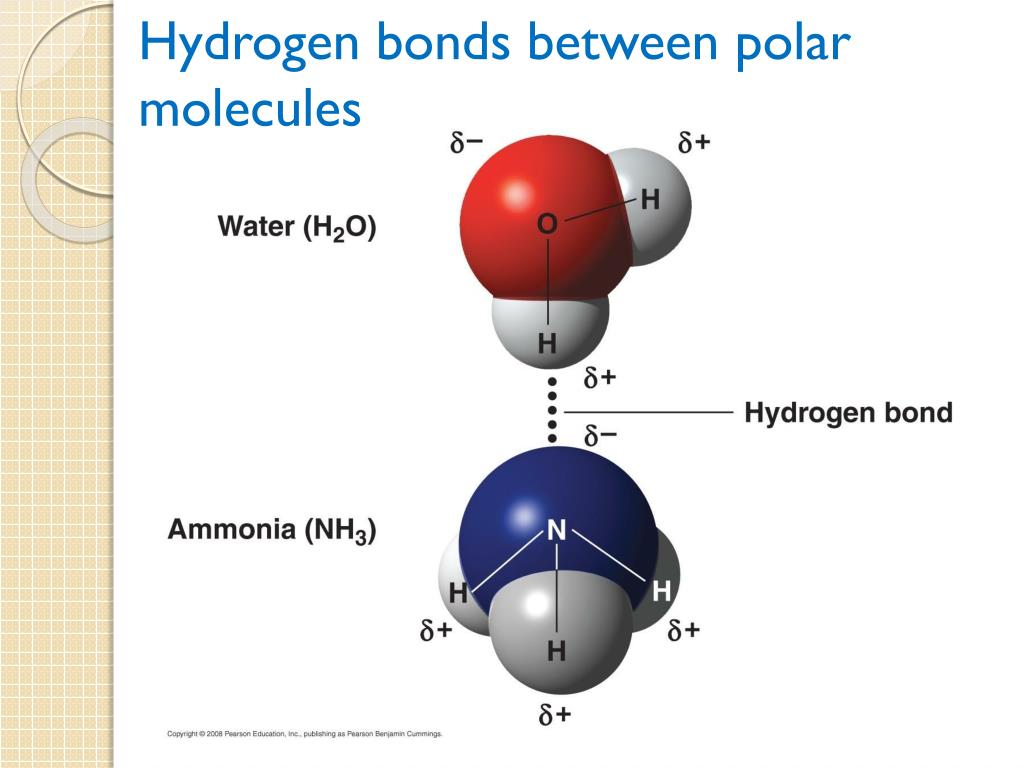
Can Polar Molecules Form Hydrogen Bonds
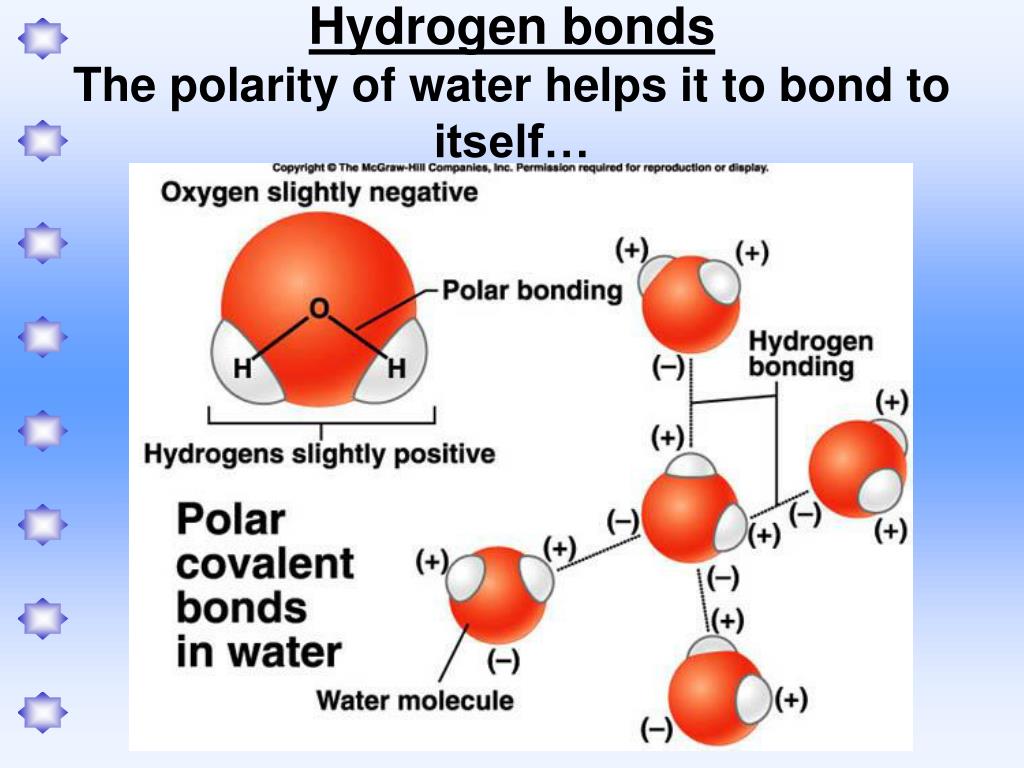
Can Polar Molecules Form Hydrogen Bonds - Hydrogen bonds are responsible for holding. When a substance readily forms hydrogen bonds with water, it can dissolve in water. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The bond is between the hydrogen of one water molecule and the oxygen atoms of another. The electronegative atoms pull on the valence electron deshields the hydrogen's proton resulting in a large δ + charge over. Hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. The hydrogen and oxygen within water molecules (h 2 o) form polar covalent bonds. These very highly electronegative elements create. Web molecules that do hydrogen bonding are always fairly polar. Web water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Web polar covalent bonds. The hydrogen and oxygen within water molecules (h 2 o) form polar covalent bonds. Water is an excellent example of hydrogen bonding. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. A typical hydrogen bond is. Web for actual hydrogen bonding, both molecules bound need to be polar. These very highly electronegative elements create. The shared electrons spend more time associated with the oxygen atom than they do with. Web this type of bond can occur in inorganic molecules such as water and in organic molecules like dna and proteins. Ad browse & discover thousands of. Water is an excellent example of hydrogen bonding. Ad browse & discover thousands of science book titles, for less. So yes, we can have hydrogen bonding. Web the result is that hydrogen forms polar covalent bonds when attached to an electronegative atom and does not form ions. Web in a water molecule adjacent to ethanol, one of the hydrogen atoms. Water is an excellent example of hydrogen bonding. So yes, we can have hydrogen bonding. Web water (h 2 o): This means the molecules will be soluble in a polar solvent such. Web in a water molecule adjacent to ethanol, one of the hydrogen atoms from the water molecule can form a hydrogen bond with the oxygen atom in the. Web the hydrogen and oxygen atoms within water molecules form polar covalent bonds. Web water (h 2 o): Hydrogen bonds are responsible for holding. The electronegative atoms pull on the valence electron deshields the hydrogen's proton resulting in a large δ + charge over. Web in a water molecule adjacent to ethanol, one of the hydrogen atoms from the water. Water is an excellent example of hydrogen bonding. Ad browse & discover thousands of science book titles, for less. Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar. Web each one of the lone pairs and the two covalently bonded hydrogen atoms can form a single hydrogen bond either with other water. This means the molecules will be soluble in a polar solvent such. Web polar covalent bonds. Web water (h 2 o): Web water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Web in some polar molecules that contain hydrogen atoms, the partial positive charge of the hydrogen atoms of one molecule are attracted to the partial negative. The electronegative atoms pull on the valence electron deshields the hydrogen's proton resulting in a large δ + charge over. Web molecules that do hydrogen bonding are always fairly polar. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Water is an excellent example of hydrogen bonding. Ad. Hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. When a substance readily forms hydrogen bonds with water, it can dissolve in water. Web the result is that hydrogen forms polar covalent bonds when attached to an electronegative atom and does not form ions. Web polar covalent. So, no, whatever interactions are happening will not be hydrogen bonds. Web this type of bond can occur in inorganic molecules such as water and in organic molecules like dna and proteins. Web in some polar molecules that contain hydrogen atoms, the partial positive charge of the hydrogen atoms of one molecule are attracted to the partial negative. The shared. Water is an excellent example of hydrogen bonding. So, no, whatever interactions are happening will not be hydrogen bonds. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Web the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons, making for a very polar bond). Web one of water’s important properties is that it is composed of polar molecules: Web the result is that hydrogen forms polar covalent bonds when attached to an electronegative atom and does not form ions. When a substance readily forms hydrogen bonds with water, it can dissolve in water. Web polar covalent bonds. Web in a water molecule adjacent to ethanol, one of the hydrogen atoms from the water molecule can form a hydrogen bond with the oxygen atom in the hydroxyl group of. Web this type of bond can occur in inorganic molecules such as water and in organic molecules like dna and proteins. So yes, we can have hydrogen bonding. Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar. The electronegative atoms pull on the valence electron deshields the hydrogen's proton resulting in a large δ + charge over. Web water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Ad browse & discover thousands of science book titles, for less. This means the molecules will be soluble in a polar solvent such. Web molecules that do hydrogen bonding are always fairly polar. Web the hydrogen and oxygen atoms within water molecules form polar covalent bonds. The shared electrons spend more time associated with the oxygen atom than they do with.covalent bond Definition, Properties, Examples, & Facts Britannica
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
PPT Chapter 2 Molecules of Life PowerPoint Presentation, free
Water has both a hydrogen bond and a polar covalent bond. Hydrogen
PPT Properties of Water PowerPoint Presentation, free download ID
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic
Chemical Bonds · Anatomy and Physiology
PPT Ch 2 The Chemical Context of Life PowerPoint Presentation, free
Hydrogen Bonding Definition, Example, Types, Question Embibe
PPT Biology Biochemistry Unit Chapter 2 The Chemistry of Life
Related Post: