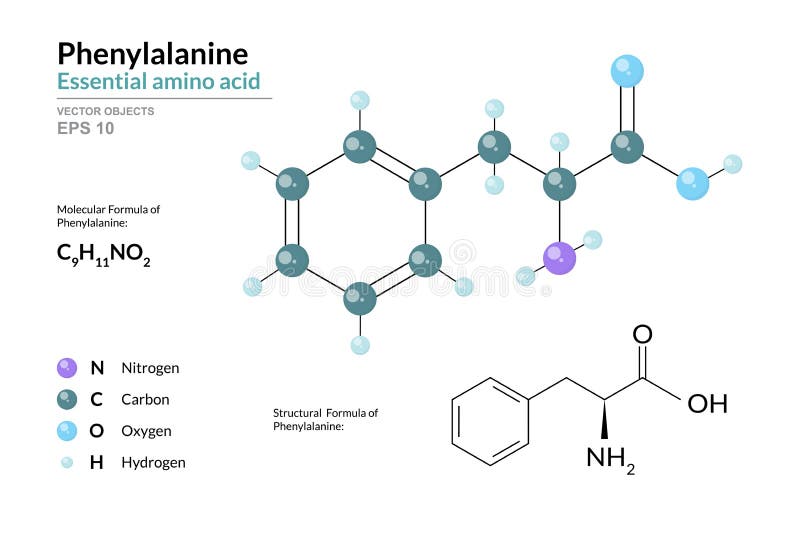
Can Phenylalanine Form Hydrogen Bonds
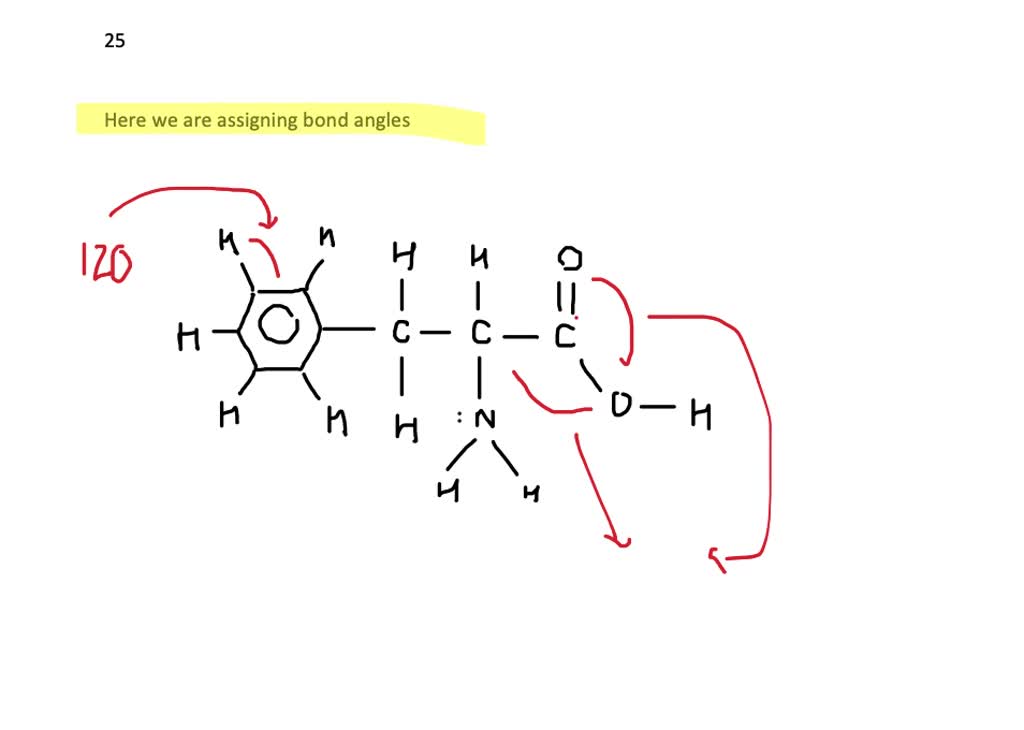
Can Phenylalanine Form Hydrogen Bonds - Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa: Web the members of the family of aromatic amino acid hydroxylases, phenylalanine hydroxylase (pheh), tyrosine hydroxylase (tyrh), and tryptophan. It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to another oxygen atom or a nitrogen atom, such as those found in polar amino acid side chains. Which of the following pairs of amino acids can form hydrogen bonds between them? However, the benzene rings in the phenylamine break. Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa: Example of salt bridge between amino acids glutamic acid and lysine demonstrating electrostatic interaction and hydrogen bonding. Note that the other three aromatics have hydrogen bonding groups at the edge of the aromatic. On the other hand, a few other. Which of the following pairs of amino acids can form hydrogen bonds between them? Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Web these hydrogen bonds therefore can give huge stabilization energy to the deformed dna as well as to the complex even. Which of the following pairs of amino acids can form hydrogen bonds between them? Y80f (1.2 a), and y86f (1.7 a). Y80f (1.2 å), and y86f (1.7 å). Web the members of the family of aromatic amino acid hydroxylases, phenylalanine hydroxylase (pheh), tyrosine hydroxylase (tyrh), and tryptophan. Only one, the one at the very top which is attached to the. Ab initio calculations are used to compare the abilities of the aromatic groups of the phe, tyr, trp, and his amino acids (modeled respectively by. Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. Note that the other three aromatics have. Web these hydrogen bonds therefore can give huge stabilization energy to the deformed dna as well as to the complex even in solution. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. It can be viewed as a benzyl group substituted for the methyl group of alanine,. Y80f (1.2 å), and y86f (1.7 å). Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. Which of the following pairs of amino acids can form hydrogen bonds between them? The structures are very similar to. Web crystal structures were determined. The structures are very similar to. Example of salt bridge between amino acids glutamic acid and lysine demonstrating electrostatic interaction and hydrogen bonding. Web phenylalanine, an amino acid present in the mixture obtained upon hydrolysis of common proteins. Y80f (1.2 a), and y86f (1.7 a). This essential amino acid is classified as neutral, and nonpolar because of the inert and. Web the members of the family of aromatic amino acid hydroxylases, phenylalanine hydroxylase (pheh), tyrosine hydroxylase (tyrh), and tryptophan. The structures are very similar to. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? The structures are very similar to. On the other hand, a few other. It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa: Ab initio calculations are used to compare the abilities of the aromatic groups of the phe, tyr,. Note that the other three aromatics have hydrogen bonding groups at the edge of the aromatic. Web due to the extended nature of the polypeptide it is not possible to form mainchain hydrogen bonds between adjacent residues. Example of salt bridge between amino acids glutamic acid and lysine demonstrating electrostatic interaction and hydrogen bonding. The structures are very similar to.. Web these hydrogen bonds therefore can give huge stabilization energy to the deformed dna as well as to the complex even in solution. The structures are very similar to. The aromatic ring is as nonpolar as benzene. However, the benzene rings in the phenylamine break. Y80f (1.2 a), and y86f (1.7 a). Web these hydrogen bonds therefore can give huge stabilization energy to the deformed dna as well as to the complex even in solution. Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. The structures are very similar to. Ab initio calculations are used to compare the abilities of the aromatic groups of the phe, tyr, trp, and his amino acids (modeled respectively by. Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Web phenylalanine, an amino acid present in the mixture obtained upon hydrolysis of common proteins. Y80f (1.2 a), and y86f (1.7 a). Web due to the extended nature of the polypeptide it is not possible to form mainchain hydrogen bonds between adjacent residues. It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The aromatic ring is as nonpolar as benzene. Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa: Y80f (1.2 å), and y86f (1.7 å). Only one, the one at the very top which is attached to the highly electrongative. Which of the following pairs of amino acids can form hydrogen bonds between them? However, the benzene rings in the phenylamine break. The structures are very similar to. Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa: On the other hand, a few other. Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to another oxygen atom or a nitrogen atom, such as those found in polar amino acid side chains.Chemical structures of the ionic forms of Lphenylalanine and of the
Structure of a fetuinbased model glycopeptide substrate docked in
Phenylalanine. Chemical Structural Formula and Model of Molecule Stock
Ch4 Polar Or Nonpolar BIOL 1124Week 2Gray Biology 1124 with
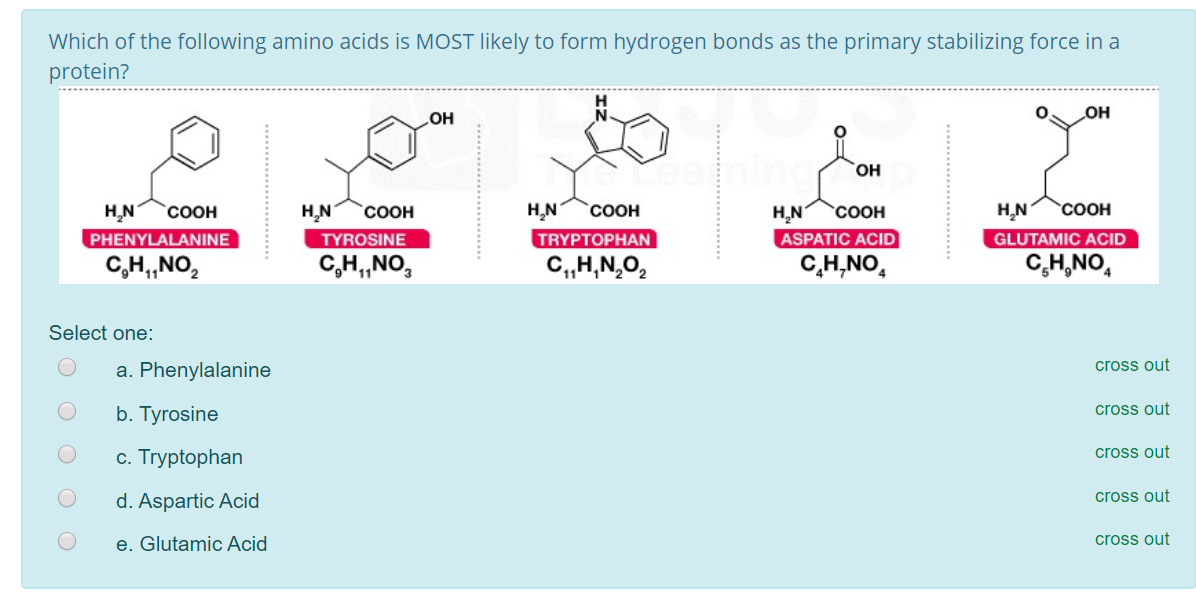
Solved Which of the following amino acids is MOST likely to
(a) Model peptides, NacetylLphenylalaninylLmethionineamide (FM
Hydrogen bonding network in HNL1 may contribute to higher... Download
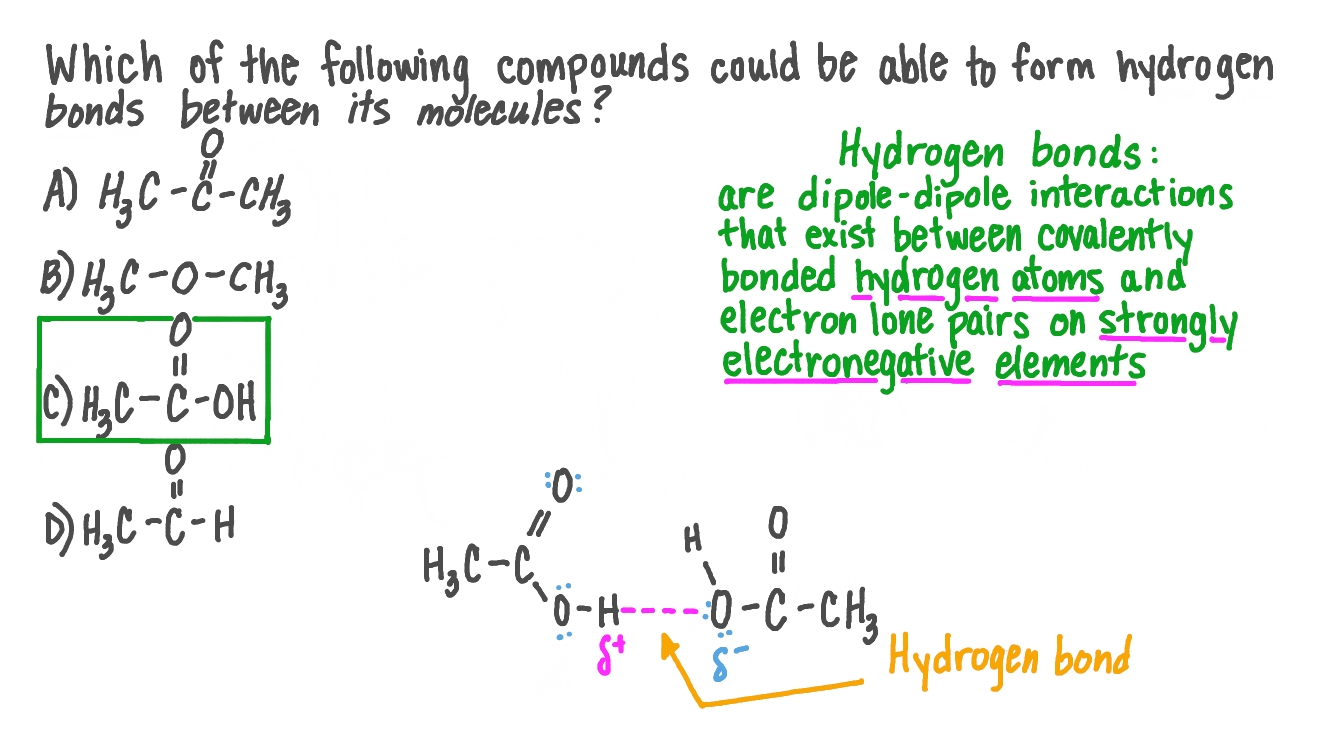
Question Video Identifying Which Compound Could Form Hydrogen Bonds
Phenylalanine is one of the natural amino acids a…
PPT Protein Structure and Enzyme Function PowerPoint Presentation
Related Post: