Can Ch4 Form Hydrogen Bonds
Can Ch4 Form Hydrogen Bonds - If we compare the boiling points of methane (ch 4 ). Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: There are exactly the right numbers of \(\delta^+\) hydrogens and lone. Web we would like to show you a description here but the site won’t allow us. H2o can form hydrogen bonds. Chemistry intermolecular bonding hydrogen bonds 1 answer anor277 · truong. Web those which do dissolve often react with the water, or else are capable of forming hydrogen bonds with the water. 8/10/2023 wiki user ∙ 8y ago study now see answers (8) best answer copy no, in ch3f all the hydrogen atoms are. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. I keep trying but fhe answer is not two, help pls :) Partial oxidation of methane to methanol (ch3oh), a more convenient, liquid fuel, is challenging because the reaction typically progresses all the way to carbon dioxide and water even with an insufficient supply of oxygen. Which of the following molecules can form hydrogen bonds? Web you'll get a detailed. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Which of the following molecules can form hydrogen bonds? Two with the hydrogen atoms and two with the with the oxygen atoms. Yes, both have an oxygen molecule for hydrogen to bond to. Chemistry intermolecular bonding hydrogen bonds 1 answer anor277 · truong. Web why are hydrogen bonds associated with water (h2o), but not with methane (ch4)? I keep trying but fhe answer is not two, help pls :) Web can ch4 for hydrogen bond updated: 4) which of the following molecules can form hydrogen bonds? Which of the following molecules can form hydrogen bonds? H2o can form hydrogen bonds. Which of the following molecules can form hydrogen bonds? Yes, both have an oxygen molecule for hydrogen to bond to. Web can ch4 for hydrogen bond updated: Partial oxidation of methane to methanol (ch3oh), a more convenient, liquid fuel, is challenging because the reaction typically progresses all the way to carbon dioxide and water even. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Two with the hydrogen atoms and two with the with the oxygen atoms. Web the correct option is d. If we compare the boiling points of methane (ch 4 ). There are exactly the right numbers of \(\delta^+\) hydrogens and. Web can ch3och3 or hcooh form hydrogen bonds with water? Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the most powerful intermolecular force influencing neutral (uncharged) molecules is the hydrogen bond. In general, methane reactions are difficult to control. I keep trying but fhe answer is not two, help pls. 4) which of the following molecules can form hydrogen bonds? As a rule of thumb, they are weaker than covalent and ionic (intramolecular) bonds, but stronger. 0/0.5 pts t question 4 how many hydrogen bonds can form between ch4 and other identical molecules? Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts.. Partial oxidation of methane to methanol (ch3oh), a more convenient, liquid fuel, is challenging because the reaction typically progresses all the way to carbon dioxide and water even with an insufficient supply of oxygen. In general, methane reactions are difficult to control. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. There are. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web the correct option is d. There are exactly the right numbers of + hydrogens. 4) which of the following molecules. Web why are hydrogen bonds associated with water (h2o), but not with methane (ch4)? Which pair of compound will form hydrogen bonds with each. Web the correct option is d. H2o can form hydrogen bonds. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Such a bond is weaker than an ionic bond or. Web can ch4 for hydrogen bond updated: 8/10/2023 wiki user ∙ 8y ago study now see answers (8) best answer copy no, in ch3f all the hydrogen atoms are. This is because hydrogen bonds are a type of electrostatic interaction, which is only possible in molecules in which. Web can ch3och3 or hcooh form hydrogen bonds with water? Two with the hydrogen atoms and two with the with the oxygen atoms. 0/0.5 pts t question 4 how many hydrogen bonds can form between ch4 and other identical molecules? H2o can form hydrogen bonds. Which of the following molecules can form hydrogen bonds? Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Chemistry intermolecular bonding hydrogen bonds 1 answer anor277 · truong. Web those which do dissolve often react with the water, or else are capable of forming hydrogen bonds with the water. Ch4 cannot form hydrogen bonds. Web the most powerful intermolecular force influencing neutral (uncharged) molecules is the hydrogen bond. There are exactly the right numbers of + hydrogens. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Why doesn't methane dissolve in water? Web we would like to show you a description here but the site won’t allow us. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts.CH4 Molecular Geometry Science Education and Tutorials
CH4 Lewis Structure, Molecular Geometry, and Hybridization
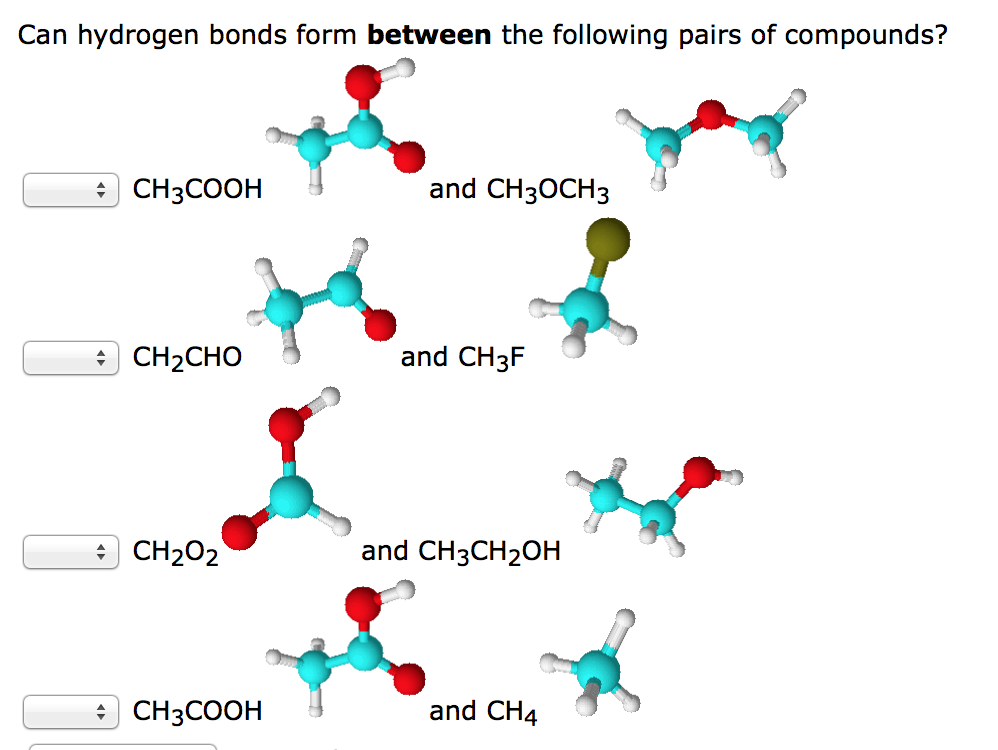
Solved Can hydrogen bonds form between the following pairs
Four covalent bonds. Carbon has four valence electrons and here a
Unit 3 Bonding Science with Dr. High
Type of Bonds for CH4 (Methane) YouTube
PPT Covalent bonding in hydrogen PowerPoint Presentation, free
Lewis Structure Ch4 Polar Or Nonpolar Oct 06, 2010 · functional
Can Ch4 Form Hydrogen Bonds CANZI
Ch4 Polar Or Nonpolar Covalent Bond Which statement explains why a
Related Post: