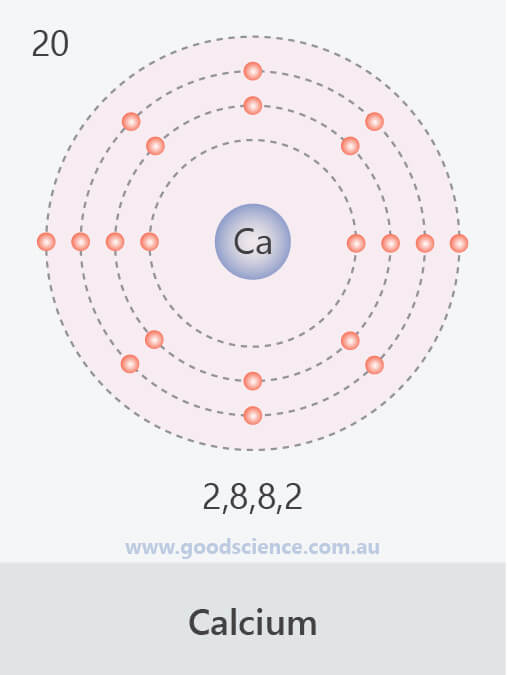
Calcium Electron Configuration Long Form
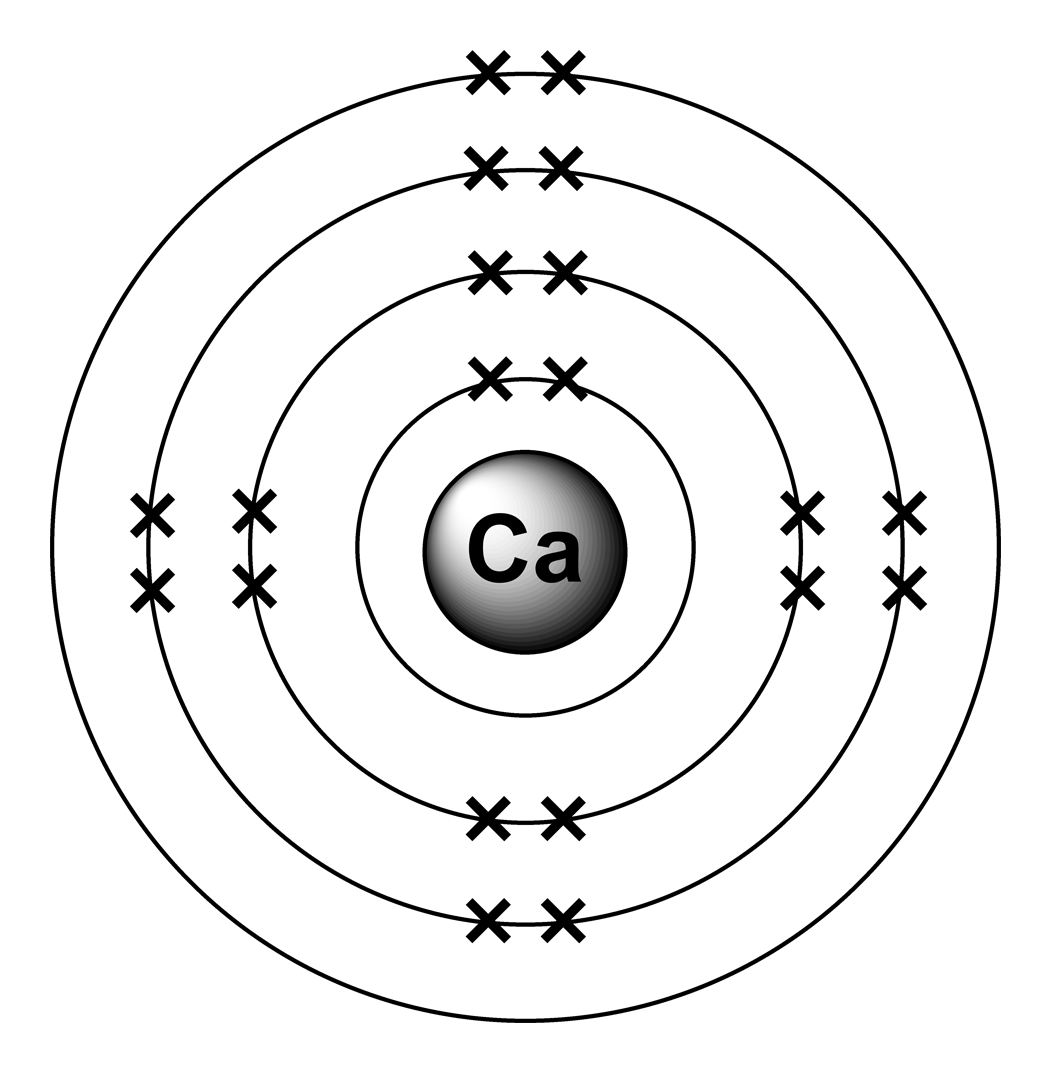
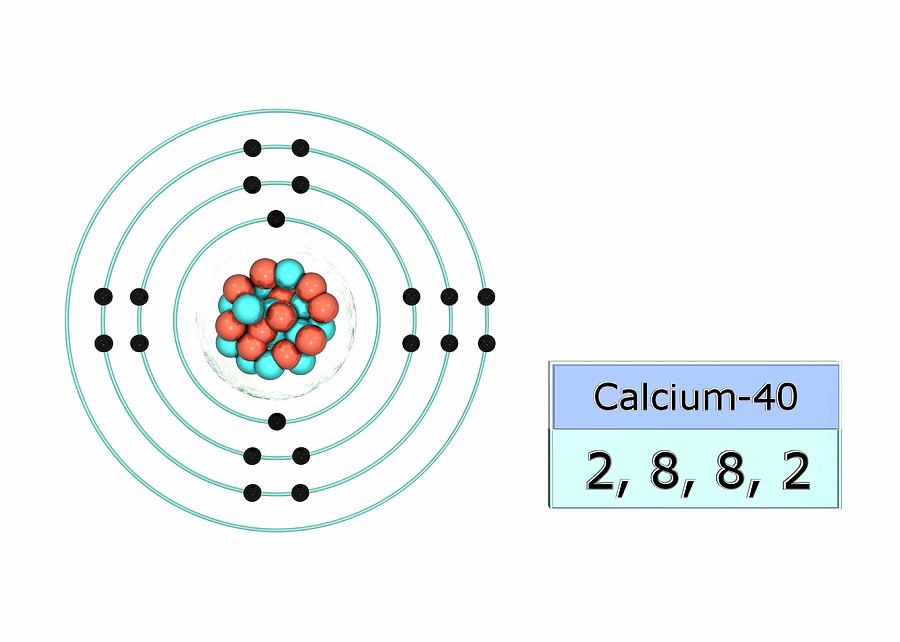
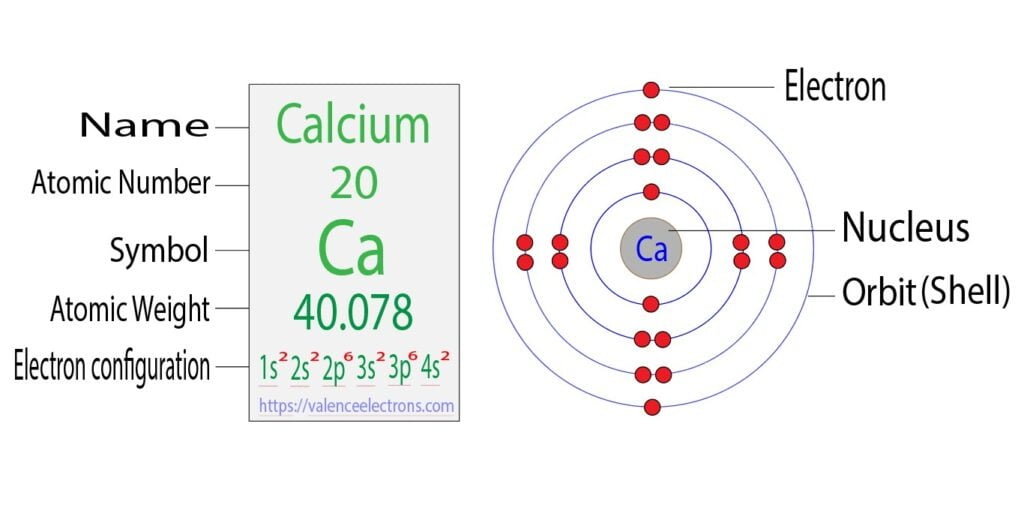
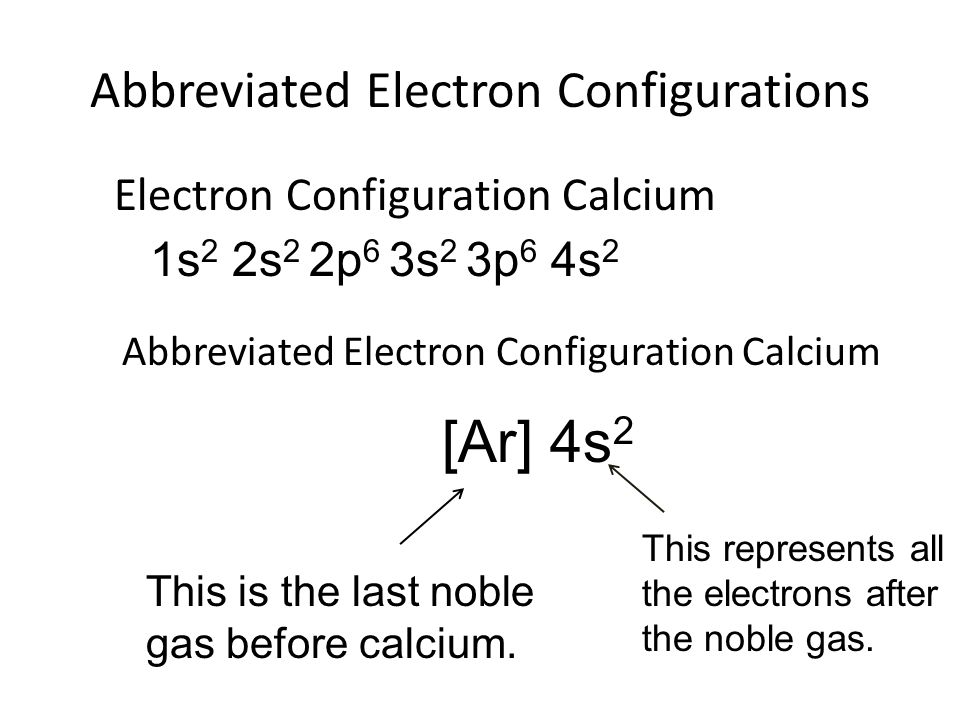
Calcium Electron Configuration Long Form - Electron configuration of fluorine (f) [he] 2s 2 2p 5: It is the fifth most abundant element in the earth’s. Davy first isolated calcium in 1808. Web what is the electron configuration of calcium? Therefore, calcium would need to gain 6 electrons in order to achieve an octet. For each atom the subshells are given first in concise form, then with all. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web hence the electron arrangement in calcium is 2, 8, 8, 2. The first two electrons in the electron configuration for calcium will be in the 1s. When we write the configuration. Calcium isotopes are 40 ca, 42 ca, 43 ca, 44 ca, 46 ca, and 48 ca. The first two electrons in the electron configuration for calcium will be in the 1s. The first ten electrons of the sodium atom are the inner. And the electron configuration of calcium is 1s2 2s2 2p6 3s2 3p6 4s2. Web the electronic configuration of. Since it is the outermost (valence) electrons. Web hence the electron arrangement in calcium is 2, 8, 8, 2. Beginning with the transition metal scandium (atomic number 21),. It is the fifth most abundant element in the earth’s. Web possible oxidation states are +2. For each atom the subshells are given first in concise form, then with all. And the electron configuration of calcium is 1s2 2s2 2p6 3s2 3p6 4s2. Web the calcium electron configuration is s² 2s² 2p⁶ 3s² 3p⁶ 4s². Web the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Davy first isolated calcium in 1808. Web what is the electron configuration of calcium? When we write the configuration. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)). Web calcium is a chemical element with the symbol ca and the atomic number 20. Beginning with the transition metal scandium (atomic number 21),. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. The first ten electrons of the sodium atom are the inner. Davy first isolated calcium in 1808. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Beginning with the transition metal scandium (atomic number 21),. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Since it is the outermost (valence) electrons. The p, d, and f orbitals have different sublevels, thus. Web possible oxidation states are +2. Therefore, calcium would need to gain 6 electrons in order to achieve an octet. The p, d, and f orbitals have different sublevels, thus. Beginning with the transition metal scandium (atomic number 21),. Davy first isolated calcium in 1808. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Chemistry electron configuration electron configuration 1 answer kris caceres jan 16, 2016 ca:[ar]4s2. Web calcium is a chemical element with the symbol ca and the atomic number 20. Web the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more. Chemistry electron configuration electron configuration 1 answer kris caceres jan 16, 2016 ca:[ar]4s2. Web the. Beginning with the transition metal scandium (atomic number 21),. The p, d, and f orbitals have different sublevels, thus. Electron configuration of fluorine (f) [he] 2s 2 2p 5: It is the fifth most abundant element in the earth’s. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)). Web the calcium electron configuration is s² 2s² 2p⁶ 3s² 3p⁶ 4s². Davy first isolated calcium in 1808. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Since it is the outermost (valence) electrons. Web hence the electron arrangement in calcium is 2, 8, 8, 2. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web calcium is a chemical element with the symbol ca and the atomic number 20. Beginning with the transition metal scandium (atomic number 21),. Web in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). For each atom the subshells are given first in concise form, then with all. Calcium isotopes are 40 ca, 42 ca, 43 ca, 44 ca, 46 ca, and 48 ca. Web what is the electron configuration of calcium? Electron configuration of fluorine (f) [he] 2s 2 2p 5: Chemistry electron configuration electron configuration 1 answer kris caceres jan 16, 2016 ca:[ar]4s2. The first two electrons in the electron configuration for calcium will be in the 1s. It is the fifth most abundant element in the earth’s. Web possible oxidation states are +2. If you want to know some other elements’ electronic configuration, you can check on our site. The p, d, and f orbitals have different sublevels, thus.Bohr Diagram Of Calcium
Which Part Of A Calcium Atom In The Ground State Heat exchanger spare
Orbital Diagram For Calcium (Ca) Calcium Electron Configuration
Calcium (Ca) electron configuration and orbital diagram (2023)
Configuration des électrons de Calcium (Ca) avec Diagramme orbital
Bohr Diagram For Calcium
Electron Configuration (Elements 120) Good Science
Calcium Facts
Orbital Diagram For Calcium Periodic Table
How to find Valency? What are valence electrons? Teachoo
Related Post: