Atoms Of Which Two Elements Will Form An Ionic Bond
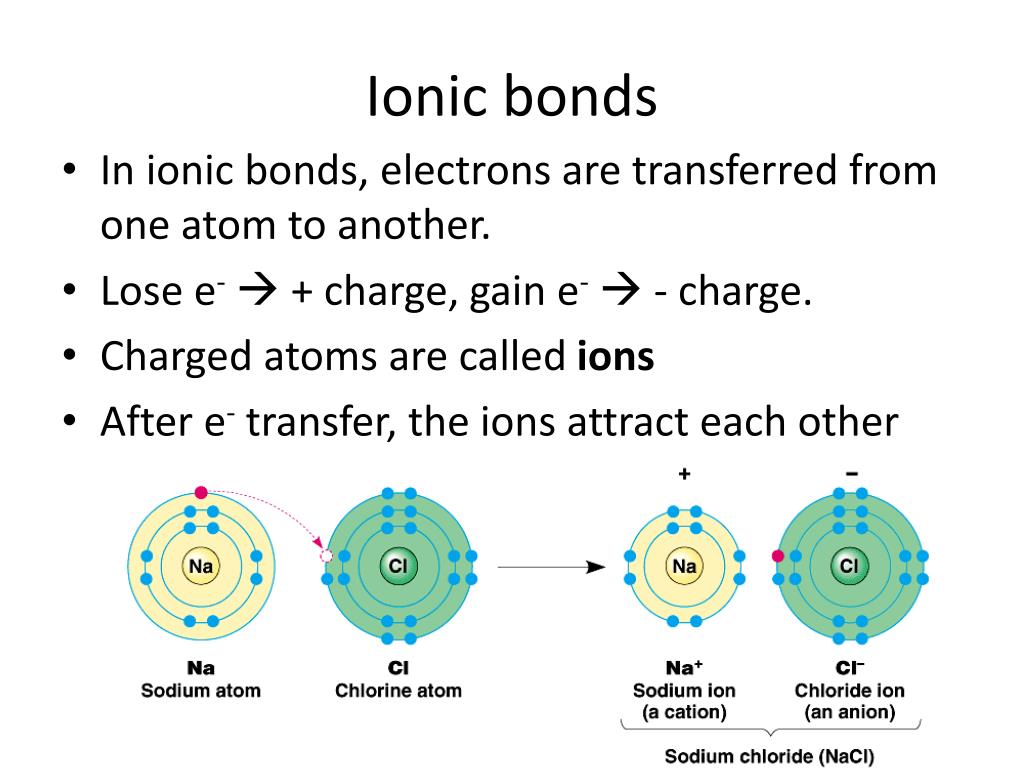
Atoms Of Which Two Elements Will Form An Ionic Bond - For example, nacl nacl is a binary. Web binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions). Ionic bonds are formed by the attraction between oppositely charged ions. Is determined by the distance at which the lowest potential energy is achieved. Let’s consider both types of. Web ions and ionic bonds. An atom that gains an electron becomes negatively charged, and is called an anion.an atom that loses. Web which elements form ionic bonds? Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 ). Calcium atom will lose two electron. For example, nacl is a binary ionic. Atoms interact with each other through the formation of chemical bonds. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is. Positive charges repel each other, so an ionic compound is not likely between two. One way to predict the type of bond that forms between two elements is to. Web binary ionic compounds are composed of just two elements: Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1. Web ions are created when an atom loses or gains an electron. The potential energy of two separate hydrogen atoms (right) decreases as. Calcium atom will lose two electron. Let’s consider both types of. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Covalent bonding involves the sharing of electrons between two or more atoms. Web ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Web in covalent bonds, two atoms. Calcium atom will lose two electron. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. When they do so, atoms form ions, or charged particles. Ad includes 5e lesson plans, reading material, quiz games, diy activities & more. Web in covalent bonds, two atoms share. An atom that gains an electron becomes negatively charged, and is called an anion.an atom that loses. Sap‑3 (eu) , sap‑3.a (lo) google classroom. Web in ionic bonding, atoms transfer electrons to each other. Web ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Web binary ionic. Web in ionic bonding, atoms transfer electrons to each other. For example, nacl is a binary ionic. Some atoms become more stable by gaining or losing an entire electron (or several electrons). A metal (which forms the cations) and a nonmetal (which forms the anions). A metal (which forms the cations) and a nonmetal (which forms the anions). One way to predict the type of bond that forms between two elements is to. Covalent bonding involves the sharing of electrons between two or more atoms. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). Web during the formation of some compounds, atoms gain or lose electrons, and. A metal (which forms the cations) and a nonmetal (which forms the anions). Covalent bonding involves the sharing of electrons between two or more atoms. Web ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Web there are primarily two forms of bonding that an atom can. When they do so, atoms form ions, or charged particles. A metal (which forms the cations) and a nonmetal (which forms the anions). Let’s consider both types of. Understand the complexities of scientific topics with amazing books. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Let’s consider both types of. Calcium atom will lose two electron. Some atoms become more stable by gaining or losing an entire electron (or several electrons). Web there are primarily two forms of bonding that an atom can participate in: In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). When they do so, atoms form ions, or charged particles. Positive charges repel each other, so an ionic compound is not likely between two. Atoms interact with each other through the formation of chemical bonds. Web binary ionic compounds are composed of just two elements: Web ions and ionic bonds. Ionic bonds require at least one electron donor and one electron acceptor. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Ionic bonds are formed by the attraction between oppositely charged ions. A metal (which forms the cations) and a nonmetal (which forms the anions). One type of chemical bond is. A metal (which forms the cations) and a nonmetal (which forms the anions). For example, nacl is a binary ionic. Web in ionic bonding, atoms transfer electrons to each other. Ionic bonds are formed by transfer of electrons between metal and non metals. Understand the complexities of scientific topics with amazing books.Ionic bonding Wikipedia
Ionic Bonding Presentation Chemistry
Ionic Bond Definition, Types, Properties & Examples
PPT Atomic structure and chemical bonding PowerPoint Presentation
ionic bond Definition, Properties, Examples, & Facts Britannica
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic Bond Definition, Types, Properties & Examples
formation of ionic bonds
What are Ionic Compounds and how they are formed?
Chemical Bonds
Related Post:

.PNG)






.PNG)