Acetylene Is Hydrogenated To Form Ethane
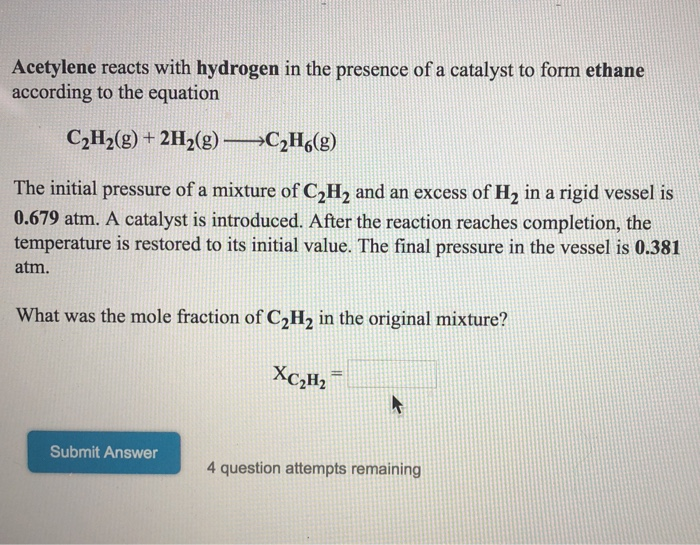
Acetylene Is Hydrogenated To Form Ethane - What is the percent yield of c2h6 for this reaction? Elementary principles of chemical processes. The feed to the reactor contains 2.25 moles of h_2 for every mole of c_2h_2. Web up to $3 cash back acetylene is hydrogenated to form ethane. 100% (1 rating) we have the balanced reaction as follows: Web over fe 2 o 3 cluster, the overall barriers for h 2 dissociation, addition reaction of two hydrogen atom are respectively 26.6, 32 and 31.8 kcal·mol −1 during the. A) write the balanced chemical reaction for this process. The feed to the reactor contains 1.4 mol h2/mol c2h2. Web the hydrogen atoms in acetylene can be replaced by metallic elements to form acetylides—e.g., acetylides of silver, copper, or sodium.the acetylides of silver,. Web up to $3 cash back acetylene, c2h2, can be converted to ethane, c2h6, by a process known as hydrogenation. A) write the balanced chemical reaction. Web the answer is usually a metal. Web acetylene is hyrodgenated to form ethane. C2h2 + 2h2 → c2h6 (a) stoichiometric ratio mol h2 react/mol c2h2 react = 2 mol h2 / 1 mol c2. Ethyne) is the chemical compound with the formula c 2 h 2 and structure h−c≡c−h.it is a hydrocarbon and. (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react) and the yield ratio (kmol c2h6 formed/kmol h2 react). The reaction proceeds to completion a. Calculate the mass feed rate of hydrogen (kg/s) required to produce 7.00 x 10 metric tons of. The feed to the reactor contains 1.50 mol h2/mol c2h2. The feed to the reactor contains 1.50. Elementary principles of chemical processes. Web acetylene is hydrogenated to form ethane. Web acetylene is hyrodgenated to form ethane. The feed to the reactor contains 1.50 mol h2 /mol c2h2. (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react) and the yield ratio (kmol c2h6 formed/kmol h2 react). The feed to the reactor contains 1.50 mol h2 /mol c2h2. 100% (1 rating) we have the balanced reaction as follows: Then, there's hydrogen, a colorless and odorless gas. The feed to the reactor contains 1.50 mol h2 / mol c2 h2 (a) calculate the stoichiometric reactant ratio (mol h2 react/mol. The feed to the reactor contains 1.5 mol h2/mol. Web acetylene is hyrodgenated to form ethane. The feed to the reactor contains 1.60 mol h2/mol c2h2. The reaction proceeds to completion. The feed to the reactor contains 1.50 mol h2 / mol c2 h2 (a) calculate the stoichiometric reactant ratio (mol h2 react/mol. A) write the balanced chemical reaction. The feed to the reactor contains 1.40 mol h2/mol c2h2. A) write the balanced chemical reaction for this process. The feed to the reactor contains 1.50 mol h2 / mol c2 h2 (a) calculate the stoichiometric reactant ratio (mol h2 react/mol. Web solved:acetylene is hydrogenated to form ethane. Web the hydrogen atoms in acetylene can be replaced by metallic elements. A) write the balanced chemical reaction. Web over fe 2 o 3 cluster, the overall barriers for h 2 dissociation, addition reaction of two hydrogen atom are respectively 26.6, 32 and 31.8 kcal·mol −1 during the. Elementary principles of chemical processes. Web acetylene is hydrogenated to form ethane. Web solved:acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.60 mol h2/mol c2h2. Elementary principles of chemical processes. Web acetylene (c2h2) is hydrogenated to form ethane (c2h6). Acetylene is hydrogenated to form ethane. Web when 28.0 grams of acetylene reacts with hydrogen, 24.5 grams of ethane is produced. Web the hydrogen atoms in acetylene can be replaced by metallic elements to form acetylides—e.g., acetylides of silver, copper, or sodium.the acetylides of silver,. Web acetylene is hydrogenated to form ethane. At least at first glance, it's not an element that suggests metallic properties. The feed to the reactor contains 1.40 mol h2/mol c2h2. Web the answer is usually a. Web over fe 2 o 3 cluster, the overall barriers for h 2 dissociation, addition reaction of two hydrogen atom are respectively 26.6, 32 and 31.8 kcal·mol −1 during the. Web determine the percentage by which acetylene is in excess. A) write the balanced chemical reaction. Write the stoichiometric equation for this reaction, and then answer the. Calculate the mass. Web determine the percentage by which acetylene is in excess. The feed to the reactor contains 1.50 mol h2 / mol c2 h2 (a) calculate the stoichiometric reactant ratio (mol h2 react/mol. Web solved:acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.50 mol h2/mol c2h2. Web acetylene is hyrodgenated to form ethane. Acetylene is hydrogenated to form ethane. The feed to the reactor contains 2.25 moles of h_2 for every mole of c_2h_2. The feed to the reactor contains 1.60 mol h2/mol c2h2. The feed to the reactor contains 2.25 moles of h 2 for every mole of c2h2. Web the answer is usually a metal. 100% (1 rating) we have the balanced reaction as follows: Web acetylene is hydrogenated to form ethane. Web over fe 2 o 3 cluster, the overall barriers for h 2 dissociation, addition reaction of two hydrogen atom are respectively 26.6, 32 and 31.8 kcal·mol −1 during the. Calculate the mass feed rate of hydrogen (kg/s) required to produce 7.00 x 10 metric tons of. The feed to the reactor contains 1.40 mol h2/mol c2h2. Web when 28.0 grams of acetylene reacts with hydrogen, 24.5 grams of ethane is produced. Web acetylene is hydrogenated to form ethane. At least at first glance, it's not an element that suggests metallic properties. What is the percent yield of c2h6 for this reaction? Elementary principles of chemical processes.Solved Acetylene reacts with hydrogen in the presence of a
Question Video Identifying the Catalyst Required for the TwoStep
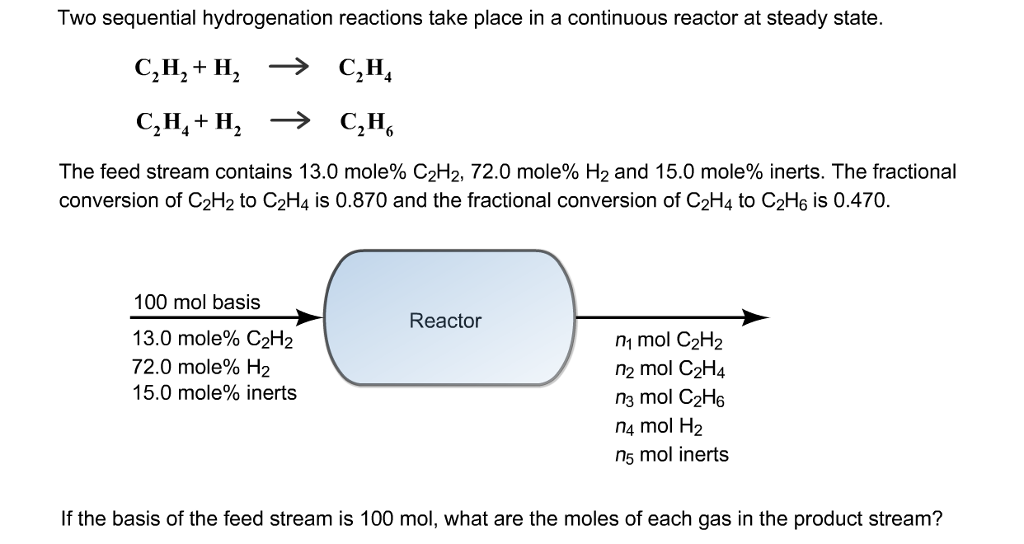
Solved The feed stream contains 13.0 mole C2H2, 72.0 mole
10ynes
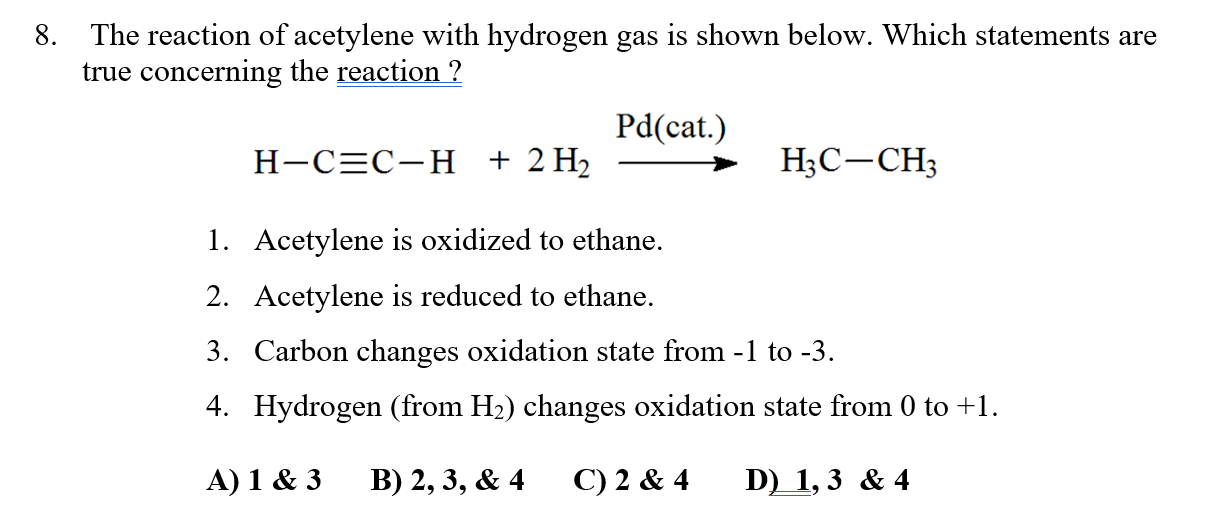
Solved 8. The reaction of acetylene with hydrogen gas is
Alkenes Chemstuff
OneClass Acetylene is hydrogenated to form ethane. The feed to the
Solved Ethyne is hydrogenated to ethene as shown in reaction
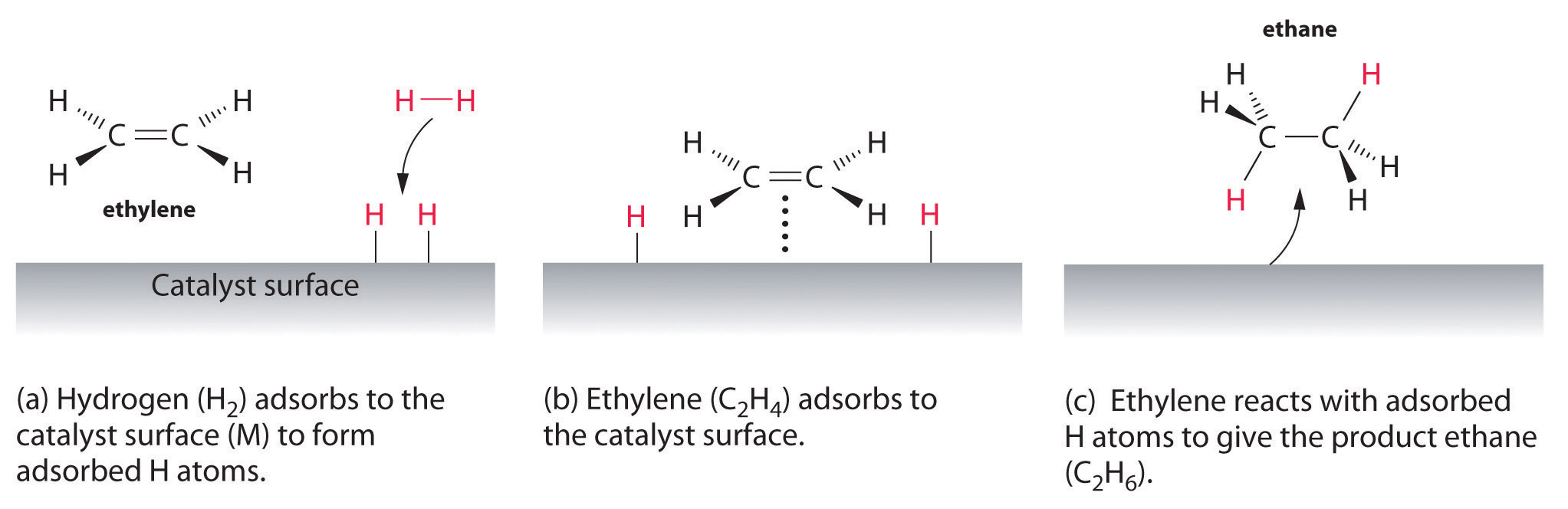
Organic Synthesis International Heterogeneous catalysis and catalyst
[Solved] Acetylene is hydrogenated to form ethane. The feed to the
Related Post: