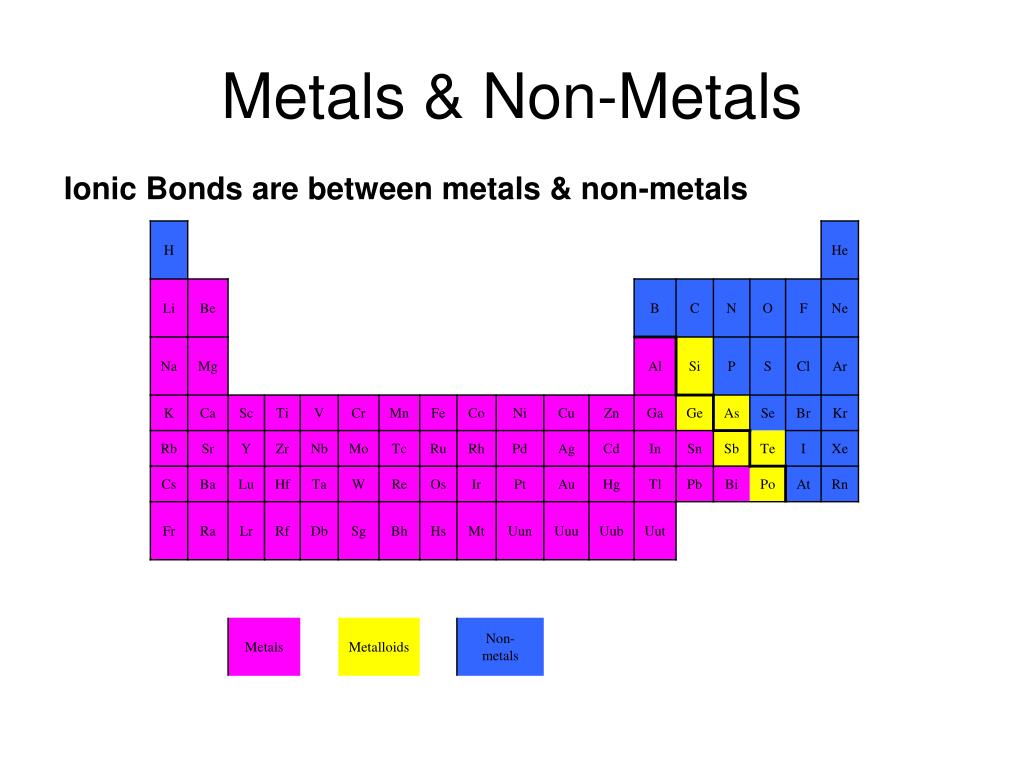
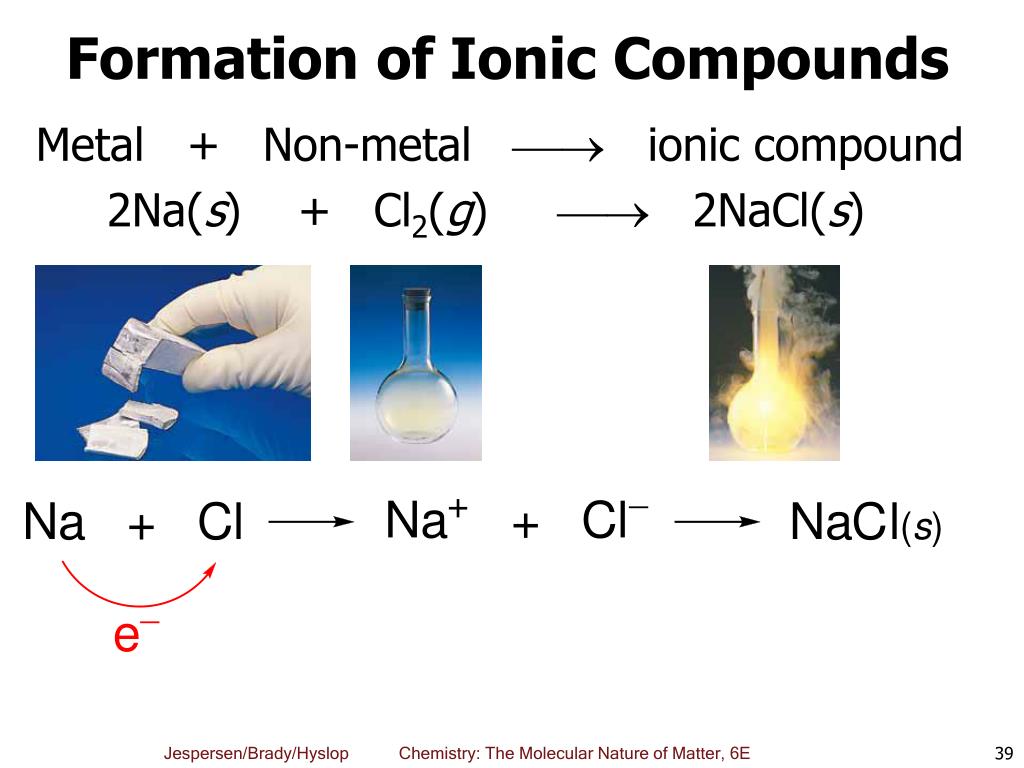
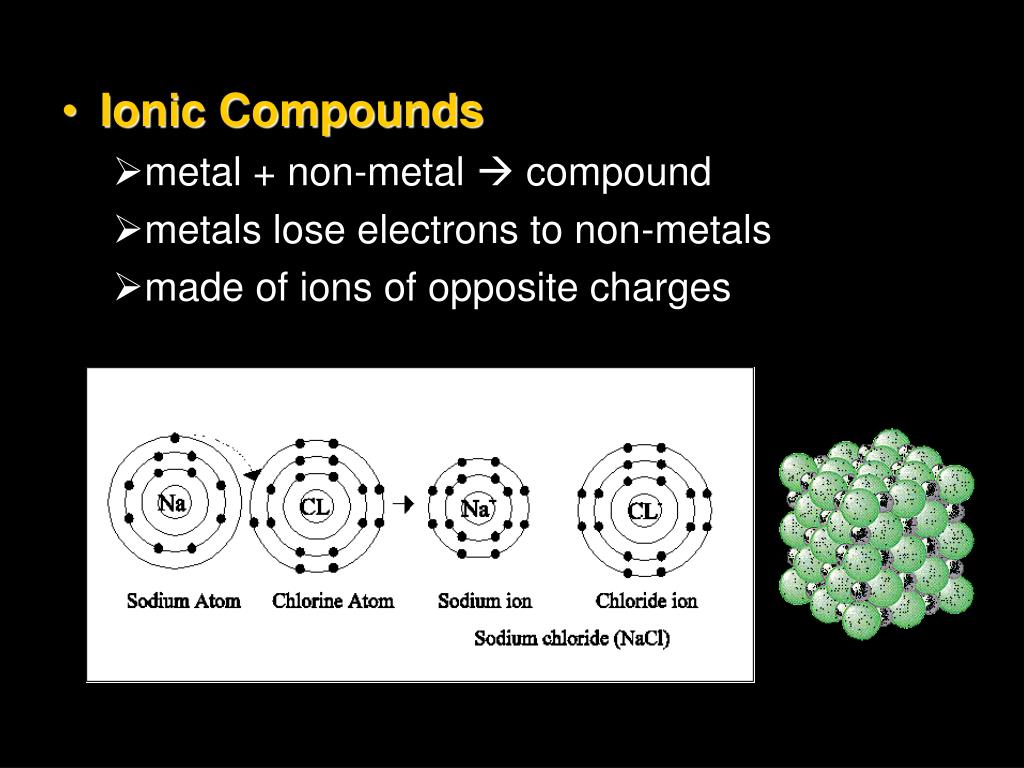
A Metal And A Nonmetal Form An Ionic Compound
A Metal And A Nonmetal Form An Ionic Compound - Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. Web nonmetals lose electrons to gain negative charge and form anions, whereas metals lose electrons to gain positive charge and form cations. Web binary ionic compounds are composed of just two elements: Ionic compounds are neutral compounds made up of positive and negative ions. Metals tend to have low. These are electronegative elements with high ionization energies. A nonmetal and another nonmetal. First, compounds between metal and nonmetal elements are usually ionic. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an electron. Part a when a metal and a nonmetal exchange electrons to make a cation and an anion, respectively, they can form an ionic. In this ocr gcse chemistry study guide, we'll go through the group 0 elements, the noble gases, are all. Ionic compounds are neutral compounds made up of positive and negative ions. Part a when a metal and a nonmetal exchange electrons to make a cation and an anion, respectively, they can form an ionic. Compounds that do not contain ions,. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an electron. First, compounds between metal and nonmetal elements are usually ionic. For example, nacl nacl is a binary. Web what are ionic compounds? In their outer shell whereas. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. A nonmetal and another nonmetal. Web ionic compounds generally form from metals and nonmetals. Identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or. Web a metal and a nonmetal. A metal (which forms the cations) and a nonmetal (which forms the anions). Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. Ionic compounds are neutral compounds made up of positive and negative ions. Web a metal and a nonmetal. Part a when a metal and a nonmetal exchange electrons to make a cation. Metals tend to have low. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. For example, nacl is a binary ionic. For example, nacl nacl is a binary. First, compounds between metal and nonmetal elements are usually ionic. Metals tend to have low. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Web what are ionic compounds? For example, nacl nacl is a binary. A nonmetal and another nonmetal. Web nonmetals lose electrons to gain negative charge and form anions, whereas metals lose electrons to gain positive charge and form cations. Web metal + nonmetal 3⁄4® ionic compound (usually) metal + polyatomic ion 3⁄4® ionic compound (usually) nonmetal + nonmetal 3⁄4® covalent compound (usually). Atoms have lots of electrons in their outer shell. Web binary ionic compounds are composed. Web metal + nonmetal 3⁄4® ionic compound (usually) metal + polyatomic ion 3⁄4® ionic compound (usually) nonmetal + nonmetal 3⁄4® covalent compound (usually). Web binary ionic compounds typically consist of a metal and a nonmetal. First, compounds between metal and nonmetal elements are usually ionic. Single and multiple covalent bonds. These are electronegative elements with high ionization energies. A metal (which forms the cations) and a nonmetal (which forms the anions). Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. For example, nacl nacl is a binary. The periodic table can help us recognize many of the compounds that are. Web there are two ways to recognize ionic compounds. Web binary ionic compounds are composed of just two elements: These two opposite ions attract each other and form the ionic bond. The periodic table can help us recognize many of the compounds that are. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an electron. Part a when a metal. The periodic table can help us recognize many of the compounds that are. Atoms have lots of electrons in their outer shell. Web there are two ways to recognize ionic compounds. Web a metal and a nonmetal. Part a when a metal and a nonmetal exchange electrons to make a cation and an anion, respectively, they can form an ionic. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. For example, nacl nacl is a binary. Web binary ionic compounds are composed of just two elements: Anions are ions that have negative. For example, cabr 2 contains a metallic element. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. Web nonmetals lose electrons to gain negative charge and form anions, whereas metals lose electrons to gain positive charge and form cations. First, compounds between metal and nonmetal elements are usually ionic. Web what are ionic compounds? A metal (which forms the cations) and a nonmetal (which forms the anions). Identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or. Web metal + nonmetal 3⁄4® ionic compound (usually) metal + polyatomic ion 3⁄4® ionic compound (usually) nonmetal + nonmetal 3⁄4® covalent compound (usually). These two opposite ions attract each other and form the ionic bond. Ionic compounds are neutral compounds made up of positive and negative ions. Web binary ionic compounds typically consist of a metal and a nonmetal.PPT Ionic Compounds Formula to Name PowerPoint Presentation, free
Chapter 2 Atoms, Molecules and Life Chemistry)
What are Ionic Compounds and how they are formed?
PPT Chapter 3 Elements, Compounds, and the Periodic Table PowerPoint
Ionic Bond Definition, Types, Properties & Examples
Question Video Determining Which Two Types of Elements Form an Ionic
PPT Molecular Compounds PowerPoint Presentation, free download ID
Examples of Ionic Compoiunds YouTube
Ionic Compounds
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173
Related Post: