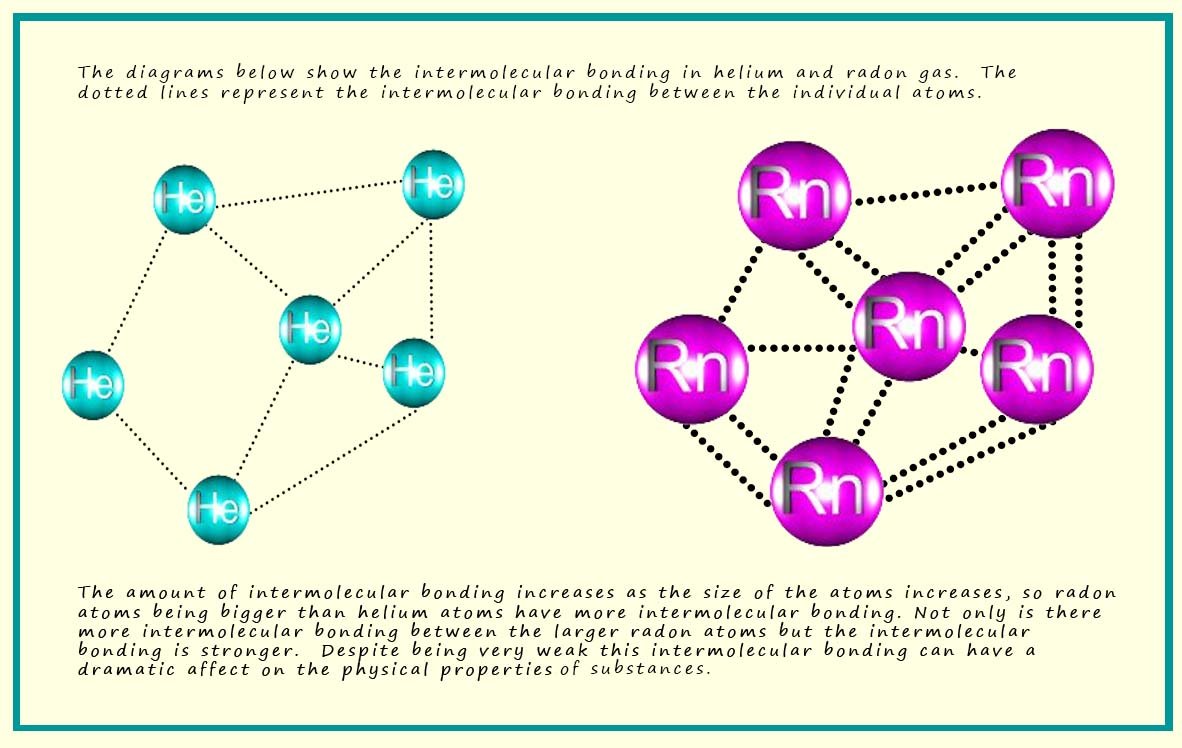
Why Don't Noble Gases Form Bonds
Why Don't Noble Gases Form Bonds - What allows metals to bond. Web why don't noble gases normally form chemical bonds. Bonds form when atoms share or transfer valence electrons. Web we would like to show you a description here but the site won’t allow us. How can chemical bonds be. Sometimes referred to as aerogens) make up a class of chemical elements with similar properties; Create an account to view solutions. Create an account to view solutions. Noble gases usually don’t form chemical bonds. Web therefore they're not going to gain anything by forming bonds. Because they have achieved a stable octet or duplet electron arrangement in their valence shell. Create an account to view solutions. Web why don't noble gases normally form chemical bonds. Web when the family of noble gases was first identified at the end of the nineteenth century, none of them were observed to form any compounds and so it was. Noble gases usually don’t form chemical bonds. In fact, they would lose something and likely have to go to a higher energy state in order to form a bond. Are special because they from a tetrahedral, hybridized orbital. Web when the family of noble gases was first identified at the end of the nineteenth century, none of them were observed. Sometimes referred to as aerogens) make up a class of chemical elements with similar properties; Web nonmetals form negative ions by (losing/gaining) enough electrons to achieve the electron configuration of the next noble gas. Web why don't noble gases normally form chemical bonds. Web therefore they're not going to gain anything by forming bonds. Web a chemical bond is a. How can chemical bonds be. Web we would like to show you a description here but the site won’t allow us. Are special because they from a tetrahedral, hybridized orbital. Sometimes referred to as aerogens) make up a class of chemical elements with similar properties; Because they have achieved a stable octet or duplet electron arrangement in their valence shell. Because they have achieved a stable octet or duplet electron arrangement in their valence shell. Create an account to view solutions. Bonds form when atoms share or transfer valence electrons. You would have been content to draw pcl 3 at. Create an account to view solutions. Create an account to view solutions. You would have been content to draw pcl 3 at. What allows metals to bond. Web when the family of noble gases was first identified at the end of the nineteenth century, none of them were observed to form any compounds and so it was initially believed that. Web why don't noble gases form. Web the noble gases (historically also the inert gases; Sometimes referred to as aerogens) make up a class of chemical elements with similar properties; Web when the family of noble gases was first identified at the end of the nineteenth century, none of them were observed to form any compounds and so it was initially believed that. Noble gases usually. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form chemical bonds because they have little tendency to gain or. Web when the family of noble gases was first identified at the end of the nineteenth century, none of them were observed to form any compounds and so it was initially believed. Web because noble gases’ outer shells are full, they are extremely stable, tending not to form chemical bonds and having a small tendency to gain or lose electrons. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form chemical bonds because they have little tendency to gain or. Web why can't noble. Web why don't noble gases form bonds? Web therefore they're not going to gain anything by forming bonds. How can chemical bonds be. Create an account to view solutions. Create an account to view solutions. Web why don't noble gases normally form chemical bonds. Web explain why noble gases are not likely to form chemical bonds. Web a chemical bond is a force of attraction between atoms or ions. What allows metals to bond. Because they have achieved a stable octet or duplet electron arrangement in their valence shell. Web we would like to show you a description here but the site won’t allow us. Web why can't noble gases form chemical bonds? Create an account to view solutions. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form chemical bonds because they have little tendency to gain or. Web because noble gases’ outer shells are full, they are extremely stable, tending not to form chemical bonds and having a small tendency to gain or lose electrons. Noble gases usually don’t form chemical bonds. In fact, they would lose something and likely have to go to a higher energy state in order to form a bond. Web when the family of noble gases was first identified at the end of the nineteenth century, none of them were observed to form any compounds and so it was initially believed that. Are special because they from a tetrahedral, hybridized orbital. Web nonmetals form negative ions by (losing/gaining) enough electrons to achieve the electron configuration of the next noble gas. Bonds form when atoms share or transfer valence electrons. Web therefore they're not going to gain anything by forming bonds. Noble gases don't form chemical bonds because they have a full outer shell (8 electrons). Create an account to view solutions. How can chemical bonds be.Describe the Properties of Noble Gases
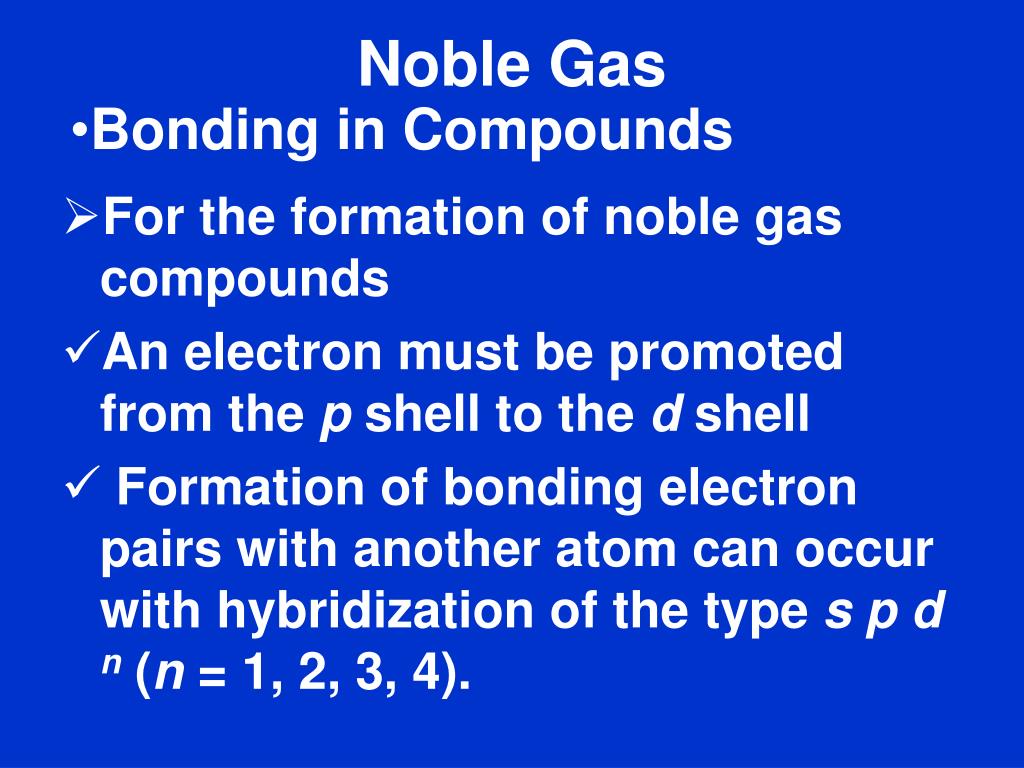
Why Atoms Make Bonds Why Noble Gases are Stable Chemical Bonding
PPT CHEMICAL BONDING PowerPoint Presentation, free download ID1711101
Noble Gas Configuration For Silicon Schott Gromentiout
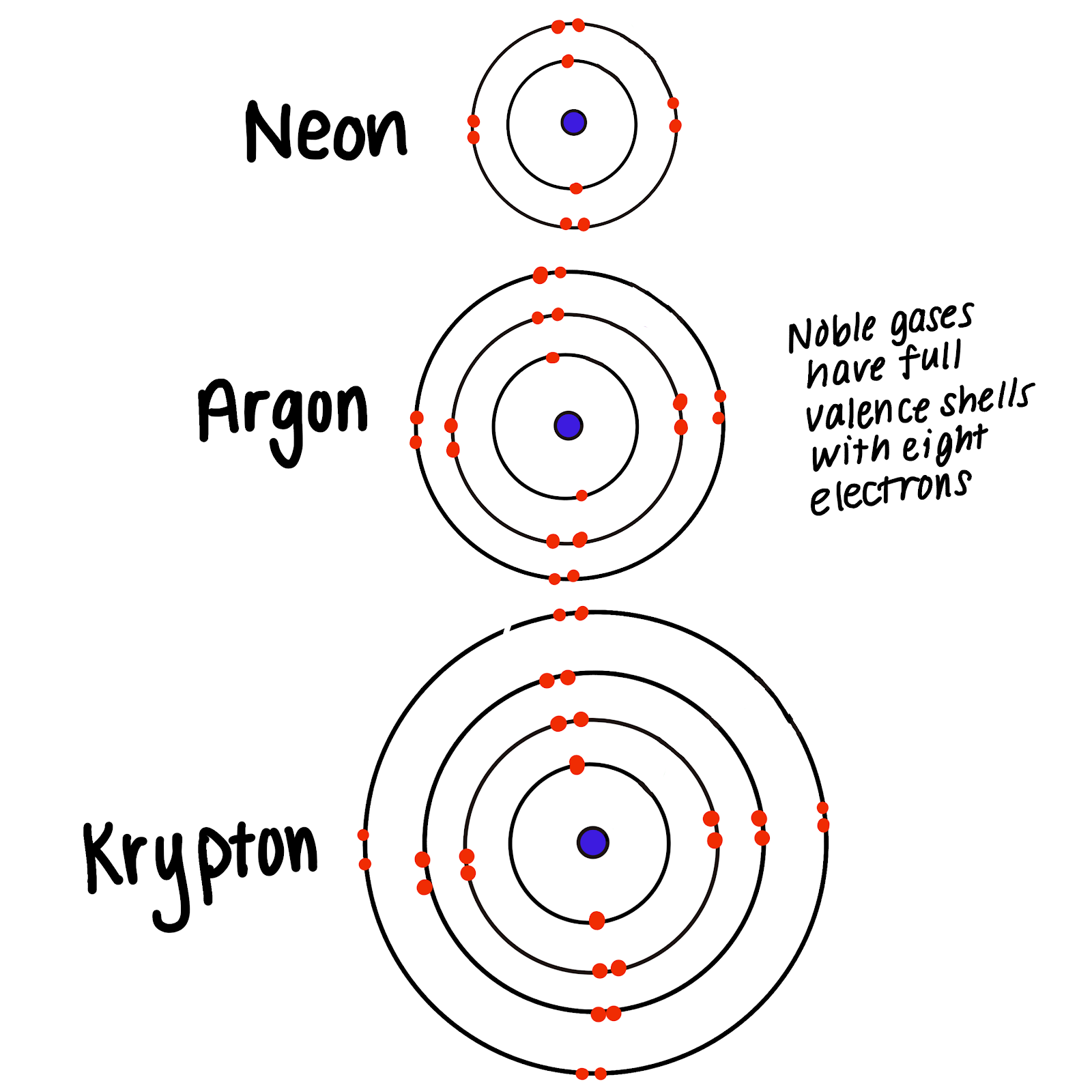
How to Write a Noble Gas Configuration for Atoms of an Element
Groups and electron dot diagrams Presentation Chemistry
The Noble gases
PPT Noble Gases PowerPoint Presentation, free download ID4507465
Why Don't Noble Gases Bond? Video & Lesson Transcript
What Are Noble Gases? Definition and Properties
Related Post:



.PNG)