Which Pair Of Elements Can Form Ionic Bonds
Which Pair Of Elements Can Form Ionic Bonds - Web the formation of ionic compounds. Is determined by the distance at which the lowest potential energy is achieved. These oppositely charged ions attract each other to form ionic networks (or lattices ). Web , sap‑3.a.3 (ek) google classroom. Web that is, group 1 elements form 1+ ions; Ionic bonds result from the attraction. Which pair of elements can form ionic bonds? See answer advertisement advertisement shemmarzan470 shemmarzan470 ionic bond. One way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. Food waste, like a feather or a bone, fall into food, causing contamination. See answer advertisement advertisement shemmarzan470 shemmarzan470 ionic bond. Web determine whether the following pairs of elements can form ionic compounds. Therefore, carbon molecules share their 4 valence electrons through single, double, and triple bonds so that each atom can achieve noble gas. Binary ionic compounds are composed of just two elements: Ionic bonding is the complete transfer of valence electron. Web there are two ways to recognize ionic compounds. Write the balance equation for the. Web the formation of ionic compounds. Binary ionic compounds are composed of just two elements: See answer advertisement advertisement shemmarzan470 shemmarzan470 ionic bond. Web that is, group 1 elements form 1+ ions; Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. A metal (which forms the cations) and a nonmetal (which forms the anions). The potential energy of two separate hydrogen atoms (right) decreases as. Web to form ionic bonds, carbon molecules must either gain. The potential energy of two separate hydrogen atoms (right) decreases as. Web there are primarily two forms of bonding that an atom can participate in: Write the balance equation for the. Web , sap‑3.a.3 (ek) google classroom. A metal (which forms the cations) and a nonmetal (which forms the anions). Atoms interact with each other through the formation of chemical bonds. Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Ad study.com has been visited by 100k+ users in the past month Web there are primarily two forms of bonding that an atom can participate in: A metal (which forms the cations) and a nonmetal. Web determine whether the following pairs of elements can form ionic compounds. Covalent bonding involves the sharing of electrons between two or more atoms. Web that is, group 1 elements form 1+ ions; Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. These oppositely charged ions attract each other to form ionic networks (or lattices. Ad study.com has been visited by 100k+ users in the past month For example, cabr 2 contains. Moving from the far right to the left on the periodic table, elements often form anions with a. First, compounds between metal and nonmetal elements are usually ionic. Atoms interact with each other through the formation of chemical bonds. First, compounds between metal and nonmetal elements are usually ionic. These oppositely charged ions attract each other to form ionic networks (or lattices ). One way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. Moving from the far right to the left on the periodic table, elements often form. Web the formation of ionic compounds. See answer advertisement advertisement shemmarzan470 shemmarzan470 ionic bond. A metal (which forms the cations) and a nonmetal (which forms the anions). Web determine whether the following pairs of elements can form ionic compounds. Atoms interact with each other through the formation of chemical bonds. Ad study.com has been visited by 100k+ users in the past month A metal (which forms the cations) and a nonmetal (which forms the anions). Binary ionic compounds are composed of just two elements: Is determined by the distance at which the lowest potential energy is achieved. A metal (which forms the cations) and a nonmetal (which forms the anions). First, compounds between metal and nonmetal elements are usually ionic. Ad study.com has been visited by 100k+ users in the past month A metal (which forms the cations) and a nonmetal (which forms the anions). Web which pair of elements that can combine to form ionic bonds. Therefore, carbon molecules share their 4 valence electrons through single, double, and triple bonds so that each atom can achieve noble gas. Atoms interact with each other through the formation of chemical bonds. Is determined by the distance at which the lowest potential energy is achieved. Web ionic bonds form between elements with very different electronegativities, resulting in transfer of electrons between the atoms. Web binary ionic compounds are composed of just two elements: The potential energy of two separate hydrogen atoms (right) decreases as. Binary ionic compounds are composed of just two elements: Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. Which pair of elements can form ionic bonds? Write the balance equation for the. See answer advertisement advertisement shemmarzan470 shemmarzan470 ionic bond. Web determine whether the following pairs of elements can form ionic compounds. Web , sap‑3.a.3 (ek) google classroom. A metal (which forms the cations) and a nonmetal (which forms the anions). Food waste, like a feather or a bone, fall into food, causing contamination. Web there are two ways to recognize ionic compounds.4.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts
Ionic Bond Definition, Types, Properties & Examples
Ionic Bond Definition, Types, Properties & Examples
ionic bond Definition, Properties, Examples, & Facts Britannica
Examples of Ionic Compoiunds YouTube
Chemical Bonds
Ionic bonding Wikipedia
Ionic Bonding Presentation Chemistry
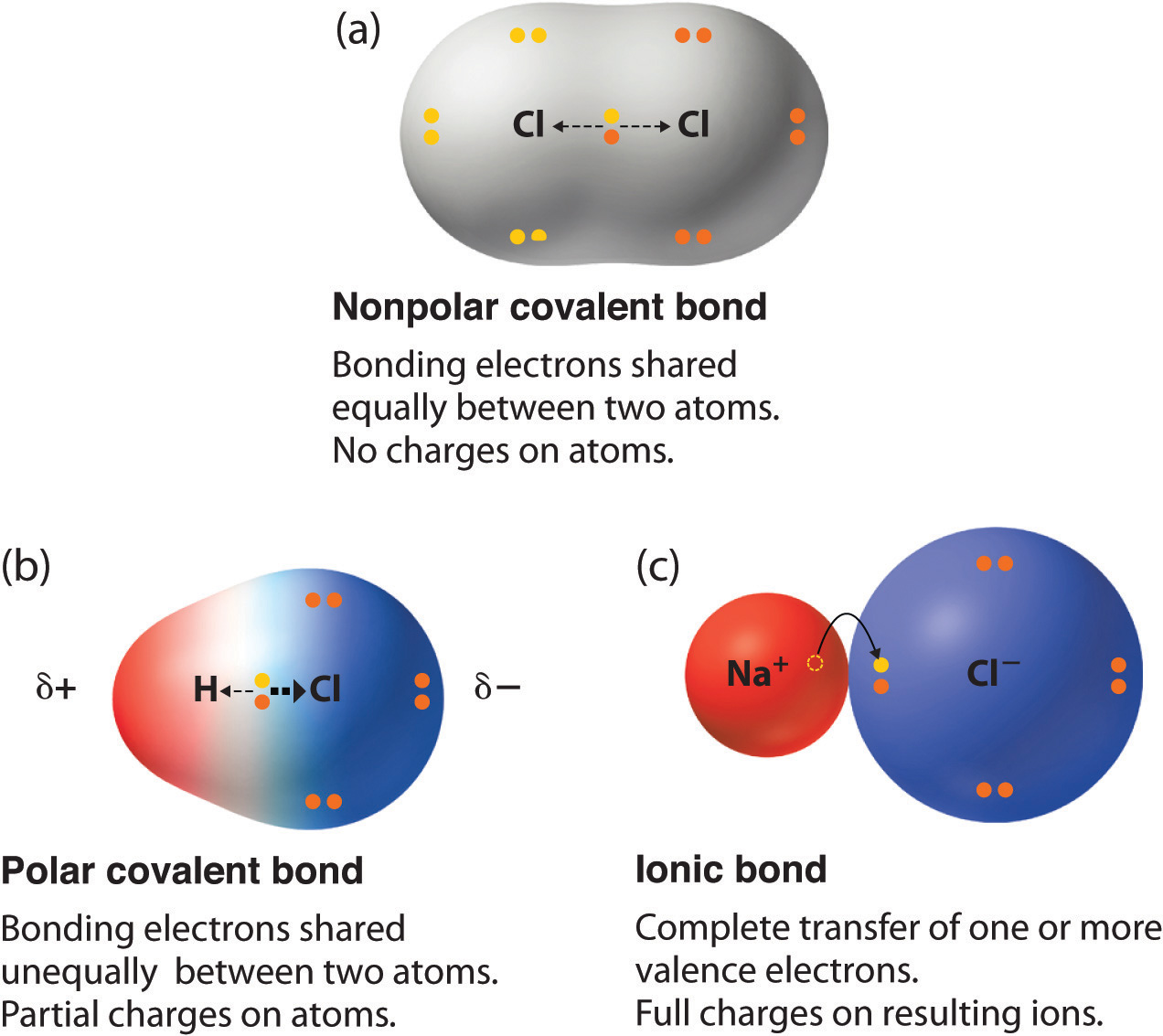
The top panel in this figure shows two hydrogen atoms sharing two
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Related Post:




.PNG)

.PNG)