Which Pair Of Atoms Will Form An Ionic Bond
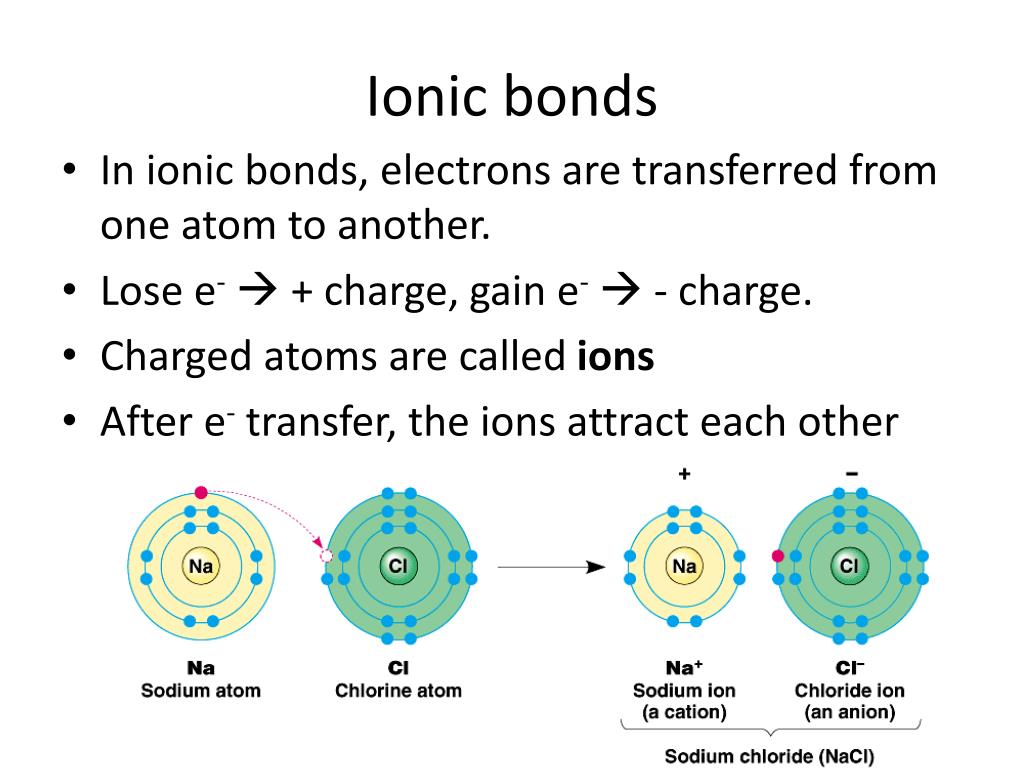
Which Pair Of Atoms Will Form An Ionic Bond - Mgt and nat get the answers you need, now! One way to predict the type of bond that forms between two elements is to. Sap‑3 (eu) , sap‑3.a (lo) , sap‑3.a.3 (ek) google classroom. If the electrons are shared equally. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. Na+ and ci o b. Get deals and low prices on chemistry atoms at amazon Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Ionic bonds are the formed by the transfer of electrons to the electronegative element from the electropositive element. Electron transfer produces negative ions called anions and positive ions. Web ionic bonds form when two or more ions come together and are held together by charge differences. The covalent bonds are formed by the equal sharing of the electrons. Na+ and ci o b. Ionic bonds are formed between cations and anions. The sodium atom transfers electrons to the chlorine atoms to form ionic bonds. Electron transfer produces negative ions called anions and positive ions. Since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web ions form when atoms gain or lose electrons. A bond distance (or bond length) is the distance between the. Web in ionic bonding, atoms transfer electrons to each other. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. From the given options, only. Web ions form when atoms gain or lose electrons. The sodium atom transfers electrons to the chlorine atoms to form ionic. Ionic bonds are the formed by the transfer of electrons to the electronegative element from the electropositive element. Electron transfer produces negative ions called anions and positive ions. One way to predict the type of bond that forms between two elements is to. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Electron transfer produces negative ions called anions and positive ions. Since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; F and ch o d. A bond distance (or bond length) is the distance. If the electrons are shared equally. Sap‑3 (eu) , sap‑3.a (lo) , sap‑3.a.3 (ek) google classroom. Web in ionic bonding, atoms transfer electrons to each other. Ionic bonds are the formed by the transfer of electrons to the electronegative element from the electropositive element. Web ionic bonds are formed when positively and negatively charged ions are held together by electrostatic. Na+ and ci o b. Consider a single pair of ions, one cation and one. Ionic bonds are formed between cations and anions. Ionic bonds are the formed by the transfer of electrons to the electronegative element from the electropositive element. Web in ionic bonding, atoms transfer electrons to each other. If the electrons are shared equally. From the given options, only. Sap‑3 (eu) , sap‑3.a (lo) , sap‑3.a.3 (ek) google classroom. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. Web a bond angle is. Mgt and nat get the answers you need, now! Ionic bonds require at least one electron donor and one electron acceptor. Sap‑3 (eu) , sap‑3.a (lo) , sap‑3.a.3 (ek) google classroom. The covalent bonds are formed by the equal sharing of the electrons. Ionic bonds are the formed by the transfer of electrons to the electronegative element from the electropositive. Na+ and ci o b. F and ch o d. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Mgt and nat get the answers you need,. Ad explore a wide variety of books in science genre from authors around the globe. Web ionic bonds form when two or more ions come together and are held together by charge differences. Sap‑3 (eu) , sap‑3.a (lo) , sap‑3.a.3 (ek) google classroom. One way to predict the type of bond that forms between two elements is to. The covalent bonds are formed by the equal sharing of the electrons. Ionic bonds are the formed by the transfer of electrons to the electronegative element from the electropositive element. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. A bond distance (or bond length) is the distance between the. Get deals and low prices on chemistry atoms at amazon From the given options, only. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Atoms of which pair of elements will form ionic bonds in a compound? Mgt and nat get the answers you need, now! Web in ionic bonding, atoms transfer electrons to each other. F and ch o d. Web ionic bonds are formed when positively and negatively charged ions are held together by electrostatic forces. Ionic bonds are formed between cations and anions. Web a bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. Electron transfer produces negative ions called anions and positive ions. Consider a single pair of ions, one cation and one.Ionic Bonding Presentation Chemistry
Examples of Ionic Bonds and Compounds
Ionic bonding Wikipedia
Ionic Bond Definition, Types, Properties & Examples
What are Ionic Compounds and how they are formed?
Chemical Bonds
PPT Atomic structure and chemical bonding PowerPoint Presentation
Molecules Compounds Bonds « KaiserScience
Ionic Bond Definition, Types, Properties & Examples
ionic bond Definition, Properties, Examples, & Facts Britannica
Related Post:
.PNG)
:max_bytes(150000):strip_icc()/ionic-bond-58fd4ea73df78ca1590682ad.jpg)



.PNG)