Which Element Will Most Likely Form Covalent Bonds With Fluorine
Which Element Will Most Likely Form Covalent Bonds With Fluorine - Which pair of elements would most likely. Web fluorine bonds with almost any element, both metals and nonmetals, because it is a very strong oxidizing agent. Web fluorine has seven electrons. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. N < c < f < o c. O < n < c< f d. Fluorine and the other halogens in group 7a (17) have seven valence. Nitrogen (n) and oxygen (o) which. Web predict the number of covalent bonds formed based on the elements involved and their position on the periodic table. Is determined by the distance at which the lowest potential energy is achieved. Web fluorine bonds with almost any element, both metals and nonmetals, because it is a very strong oxidizing agent. Web this problem has been solved! Web thegreatkeniaalejo top creator on quizlet terms in this set (10) which nonmetal would an ion of ca2+ most likely ionically bond with in a 1:1 ratio? O < n < c< f d. It. Based on this information, which element is most likely to form a strong ionic bond. Describe the important exceptions to the octet rule. Web an element that fluorine will form a covalent bond with. N < c < f < o c. In contrast, the diatomic molecules of the neighboring element oxygen, with two unpaired electrons per molecule, are paramagnetic. Web an element that fluorine will form a covalent bond with. Is determined by the distance at which the lowest potential energy is achieved. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. This makes it diamagnetic (slightly repelled by magnets) with the magnetic susceptibility of −1.2×10 (si), which is close to. F < n < c < o a which of the following elements has the highest electronegativity? Web carbon will bind covalently with fluorine (to form carbon tetrafluoride) with each of the electrons on the outermost shell of the carbon been shared covalently with. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)),. Describe the important exceptions to the. Web fluorine bonds with almost any element, both metals and nonmetals, because it is a very strong oxidizing agent. Describe the important exceptions to the octet rule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web carbon will bind covalently with fluorine (to form carbon tetrafluoride) with each of the electrons on. Web based on their locations in the periodic table, which two elements are most likely to form covalent bonds with each other? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. It is very unstable and reactive since it is so. Web which combinations of the elements would be most likely to form. Web an element that fluorine will form a covalent bond with. Web this image shows electronegativities of elements on the periodic table. Fluorine will form covalent and ionic bonds. It is very unstable and reactive since it is so. Web predict the number of covalent bonds formed based on the elements involved and their position on the periodic table. Is determined by the distance at which the lowest potential energy is achieved. Web fluorine has seven electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Describe the important exceptions to the octet rule. C < n < o < f b. Fluorine will form covalent and ionic bonds. It is very unstable and reactive since it is so. Based on this information, which element is most likely to form a strong ionic bond. Fluorine is a halide and forms covalent bonds of the functional group halides. Like in sf6, sulfur can bond with 6 fluorine atoms, due to additional d orbitals. Nitrogen (n) and oxygen (o) which. Fluorine and the other halogens in group 7a (17) have seven valence. When fluorine reacts with aluminium, is formed.since is metal and is non metallic,the compound has ionic nature. Web carbon will bind covalently with fluorine (to form carbon tetrafluoride) with each of the electrons on the outermost shell of the carbon been shared. Describe the important exceptions to the octet rule. Web fluorine has seven electrons. Web based on their locations in the periodic table, which two elements are most likely to form covalent bonds with each other? In contrast, the diatomic molecules of the neighboring element oxygen, with two unpaired electrons per molecule, are paramagnetic (attracted to magnets). Is determined by the distance at which the lowest potential energy is achieved. This makes it diamagnetic (slightly repelled by magnets) with the magnetic susceptibility of −1.2×10 (si), which is close to theoretical predictions. O < n < c< f d. Which pair of elements would most likely. Web covalent bonds between different atoms. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)),. Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between. Web an element that fluorine will form a covalent bond with. When fluorine reacts with aluminium, is formed.since is metal and is non metallic,the compound has ionic nature. Fluorine and the other halogens in group 7a (17) have seven valence. Web carbon will bind covalently with fluorine (to form carbon tetrafluoride) with each of the electrons on the outermost shell of the carbon been shared covalently with. N < c < f < o c. Web thegreatkeniaalejo top creator on quizlet terms in this set (10) which nonmetal would an ion of ca2+ most likely ionically bond with in a 1:1 ratio? Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. It is very unstable and reactive since it is so. Based on this information, which element is most likely to form a strong ionic bond.Covalent bonds Learning Lab
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
PPT Chemical Bonding and Nomenclature PowerPoint Presentation, free
Covalent Bonding (Biology) — Definition & Role Expii
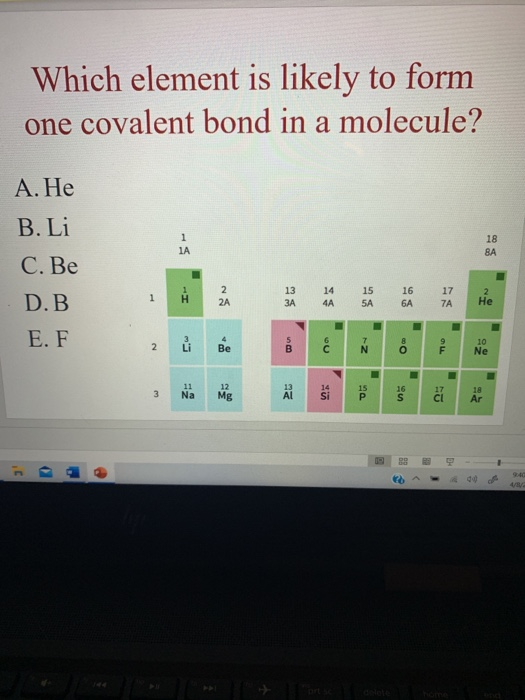
Solved Which element is likely to form one covalent bond in
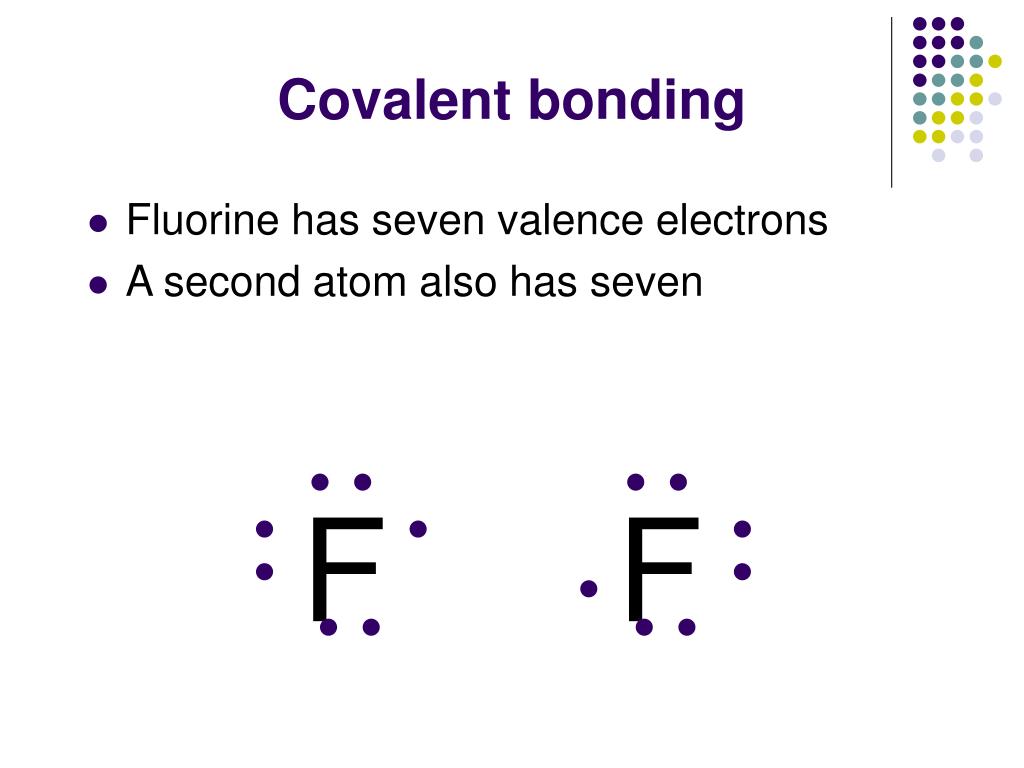
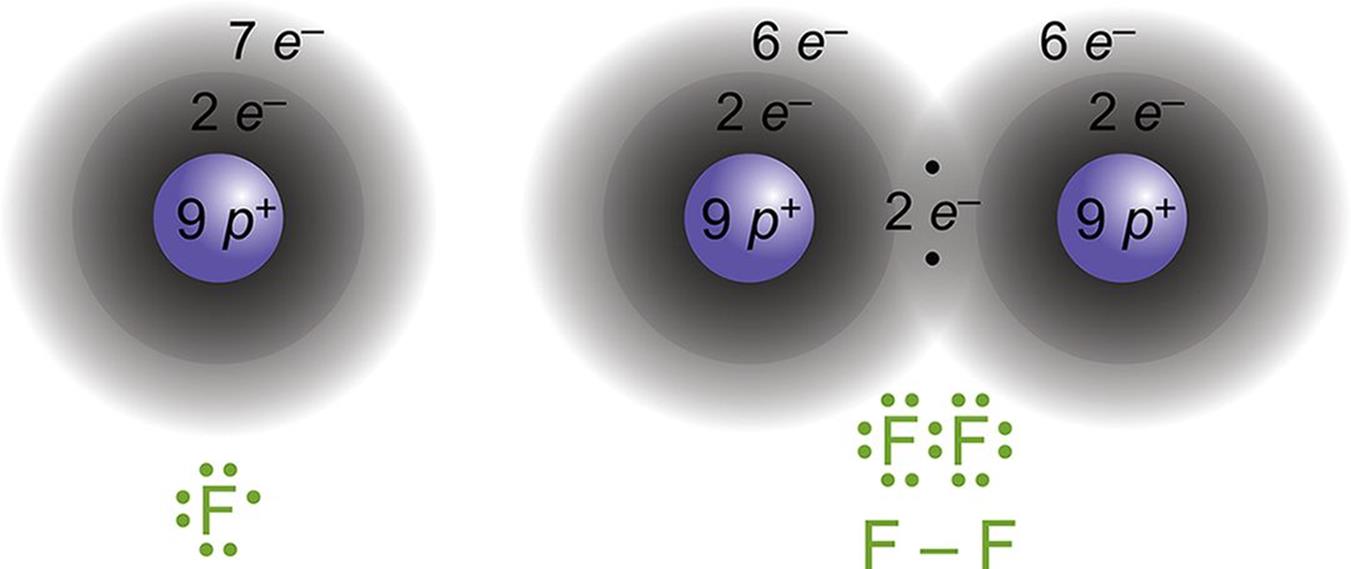
Figure 3.4. Formation of a Covalent Bond Fluorine (F) has seven valence
PPT Ionic Bonding L.O. PowerPoint Presentation, free download ID
Covalent Bonding (Molecules) Presentation Chemistry
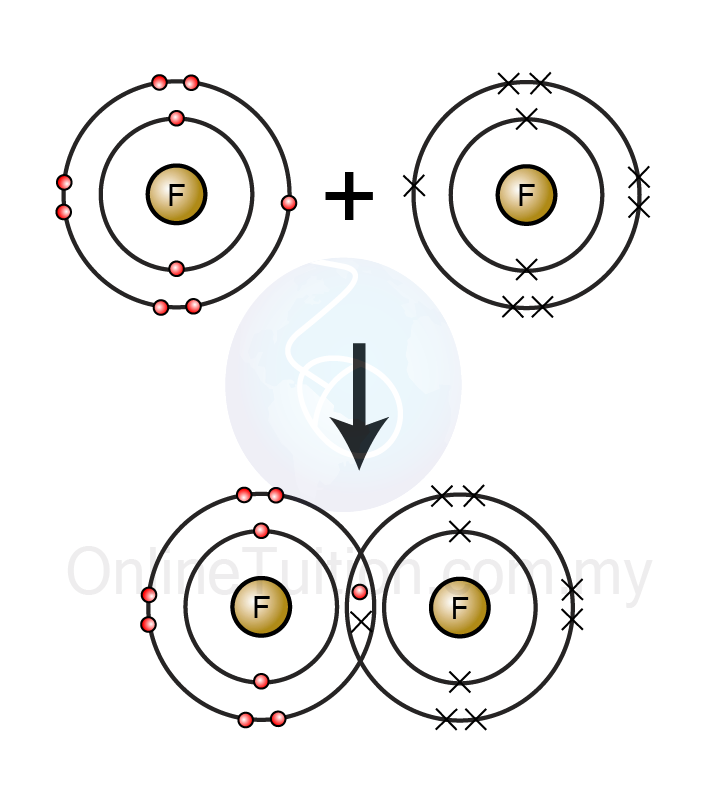
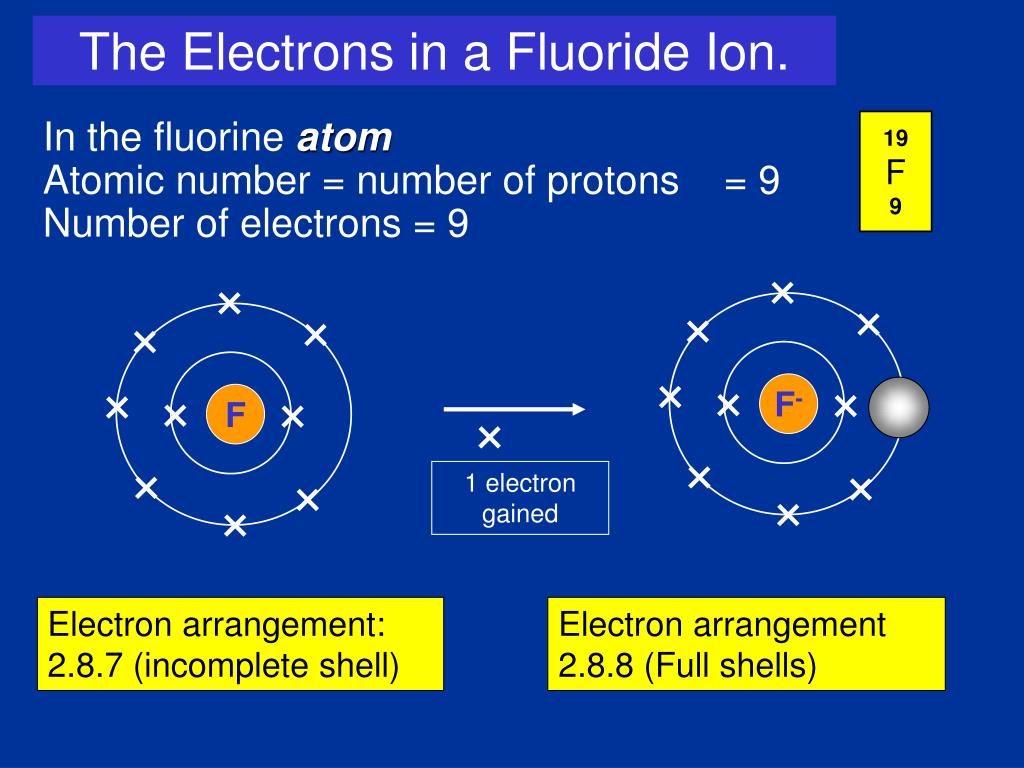
What occurs as two atoms of fluorine combine to a molecule of
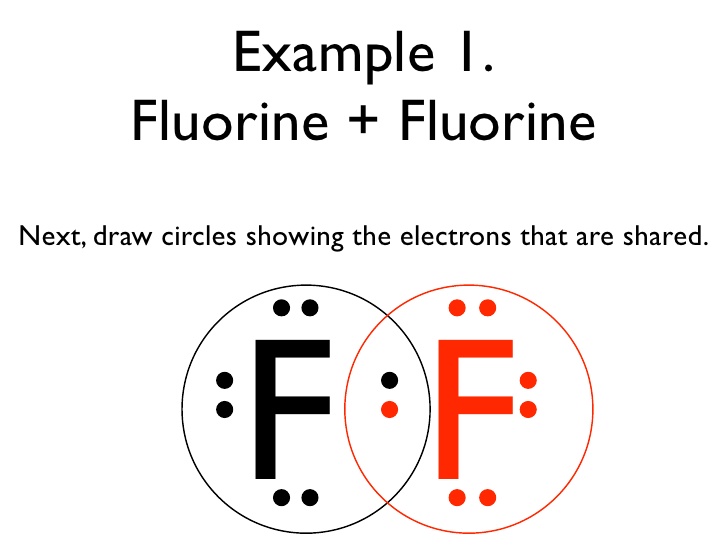
Covalent Bonding SPM Chemistry
Related Post:



.PNG)