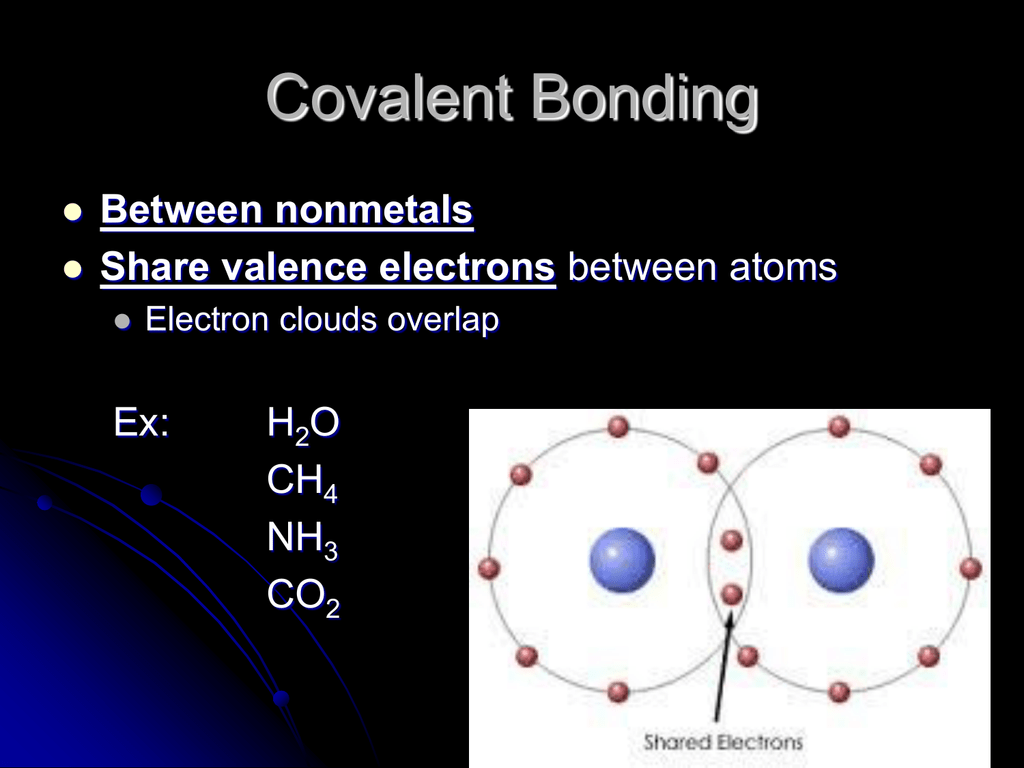
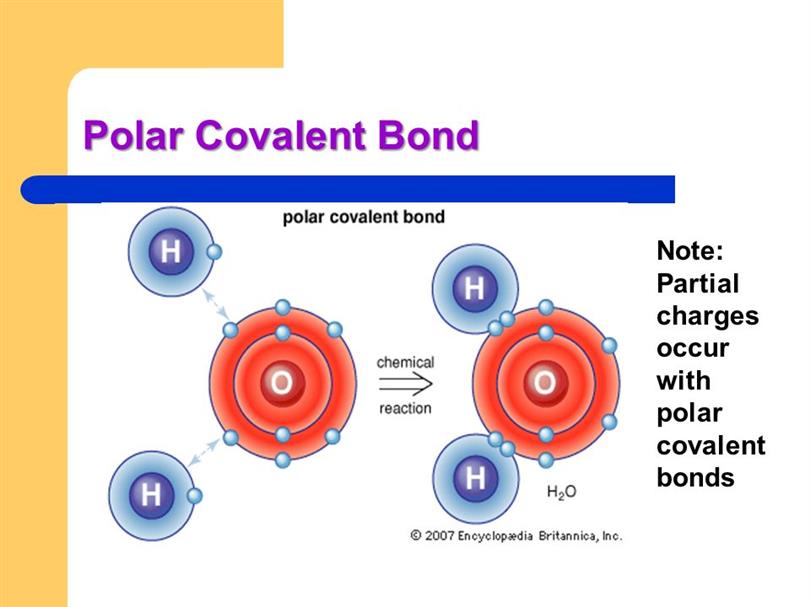
Which Combination Of Atoms Can Form A Polar Covalent Bond
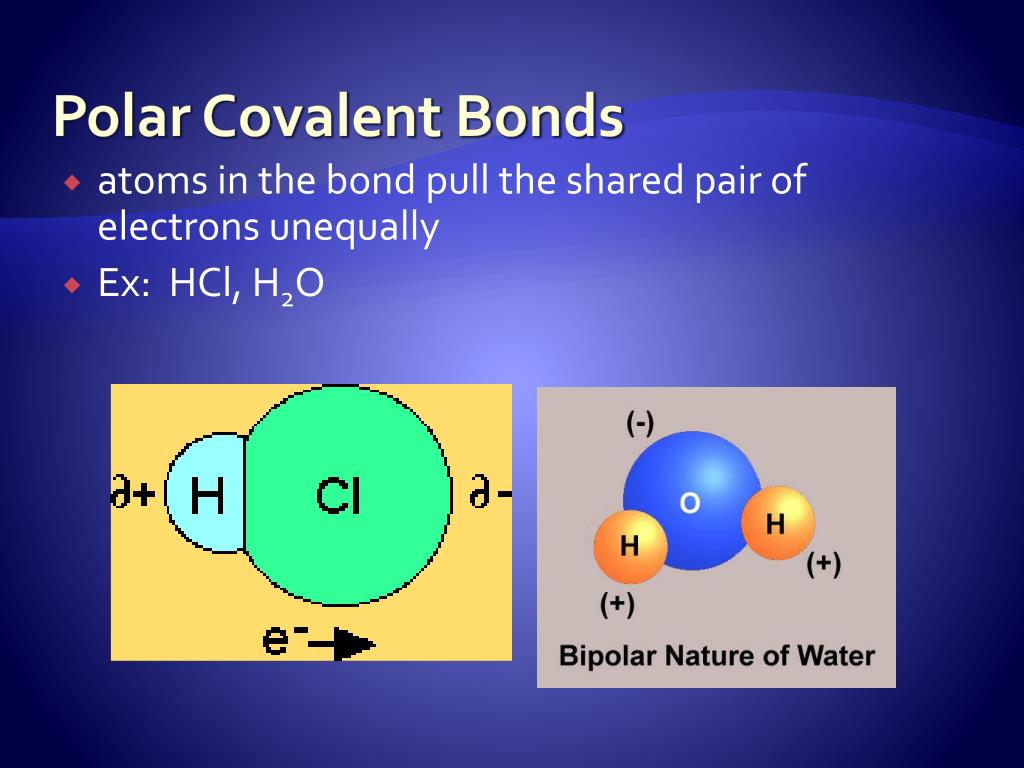
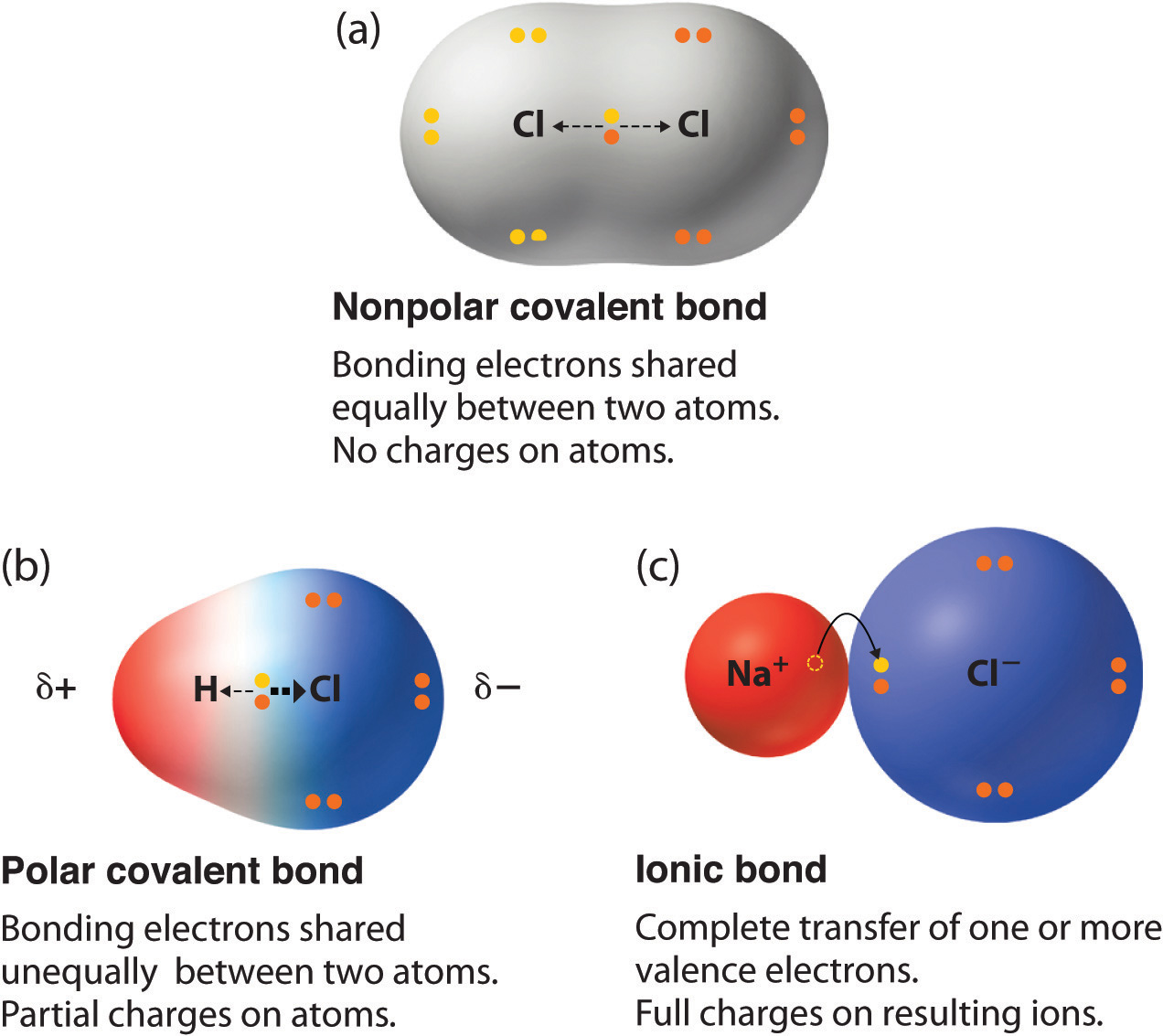
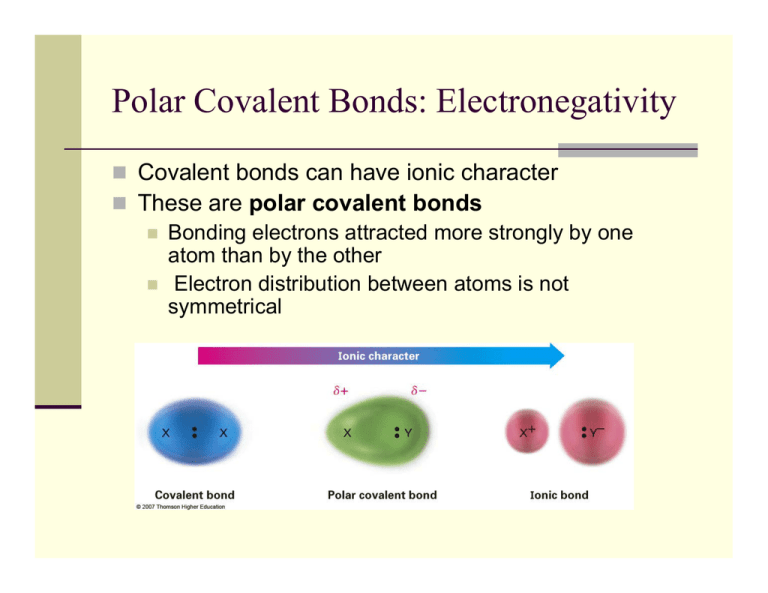
Which Combination Of Atoms Can Form A Polar Covalent Bond - When atoms bond together, they create molecules: Is determined by the distance at which the lowest potential energy is achieved. Web a coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. Some bonds between different elements are only minimally polar,. Bond in which one or more pairs of electrons are shared by two atoms. Bond in which one or more. Web the two extreme cases of chemical bonds are: Web typically, the atoms of group 4a form 4 covalent bonds; Web two hydrogen atoms can combine by donating each of their electrons into a single covalent bond, depicted on the right as the area where the gray clouds around each hydrogen. Web a polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. Web any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Web the bond length is determined by the distance at which the lowest potential energy is achieved. Classify different types of atomic bonds. Web a polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond.. Ad browse & discover thousands of science book titles, for less. However, these polyatomic ions form ionic compounds by combining. Bond in which one or more. Conversely, the same amount of energy is released when one mole of h 2 molecules forms from two moles of h atoms: These electron pairs are known as shared pairs or. Ad study.com has been visited by 100k+ users in the past month Web a polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. What you’ll learn to do: Bond in which one or more. Group 6a form 2 bonds; The potential energy of two separate hydrogen. Ad study.com has been visited by 100k+ users in the past month Consider the hydrogen chloride (hcl) molecule. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. However, these polyatomic ions form ionic compounds by combining. A polar bond forms when shared electrons are pulled closer to one. Web typically, the atoms of group 4a form 4 covalent bonds; Conversely, the same amount of energy is released when one mole of h 2 molecules forms from two moles of h atoms: And group 7a form one bond. Web a covalent bond is a chemical bond that. 2h ( g) h 2 ( g) δ h =. When atoms bond together, they create molecules: Some bonds between different elements are only minimally polar,. Web polar covalent bond is defined as type of chemical bond where a pair of electrons is unequally shared between the two atoms due to the difference in their. Bond in which one or. Bond in which one or more. Some bonds between different elements are only minimally polar,. Which combination of atoms can form a polar. The potential energy of two separate hydrogen. Group 6a form 2 bonds; Is determined by the distance at which the lowest potential energy is achieved. When atoms bond together, they create molecules: Group 5a form 3 bonds; Conversely, the same amount of energy is released when one mole of h 2 molecules forms from two moles of h atoms: Bond in which one or more. 2h ( g) h 2 ( g) δ h =. However, these polyatomic ions form ionic compounds by combining. Web atoms that gain electrons become negatively charged, while those that lose electrons become positive. Some bonds between different elements are only minimally polar,. Classify different types of atomic bonds. Web there are two basic types of covalent bonds: 2h ( g) h 2 ( g) δ h =. Classify different types of atomic bonds. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web a polar covalent bond exists when atoms with different electronegativities share electrons in a. However, these polyatomic ions form ionic compounds by combining. Some bonds between different elements are only minimally polar,. Classify different types of atomic bonds. Web the bond length is determined by the distance at which the lowest potential energy is achieved. Web atoms that gain electrons become negatively charged, while those that lose electrons become positive. Web two hydrogen atoms can combine by donating each of their electrons into a single covalent bond, depicted on the right as the area where the gray clouds around each hydrogen. Is determined by the distance at which the lowest potential energy is achieved. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Group 6a form 2 bonds; Web a polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be. Web any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Consider the hydrogen chloride (hcl) molecule. 2h ( g) h 2 ( g) δ h =. Web polar covalent bond is defined as type of chemical bond where a pair of electrons is unequally shared between the two atoms due to the difference in their. A polar bond forms when shared electrons are pulled closer to one. And group 7a form one bond. Web h 2 ( g) 2 h ( g) δ h = 436 kj. Covalent bonding occurs when pairs of electrons are shared by atoms. Ad study.com has been visited by 100k+ users in the past monthWhat Is a Polar Bond? Definition and Examples
How does a polar bond differ from a covalent bond
PPT Bond Polarity and Molecules PowerPoint Presentation, free
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Polar Covalent Bonds
Polar Covalent Bonds Electronegativity
Polar vs. Nonpolar Bonds — Overview & Examples Expii
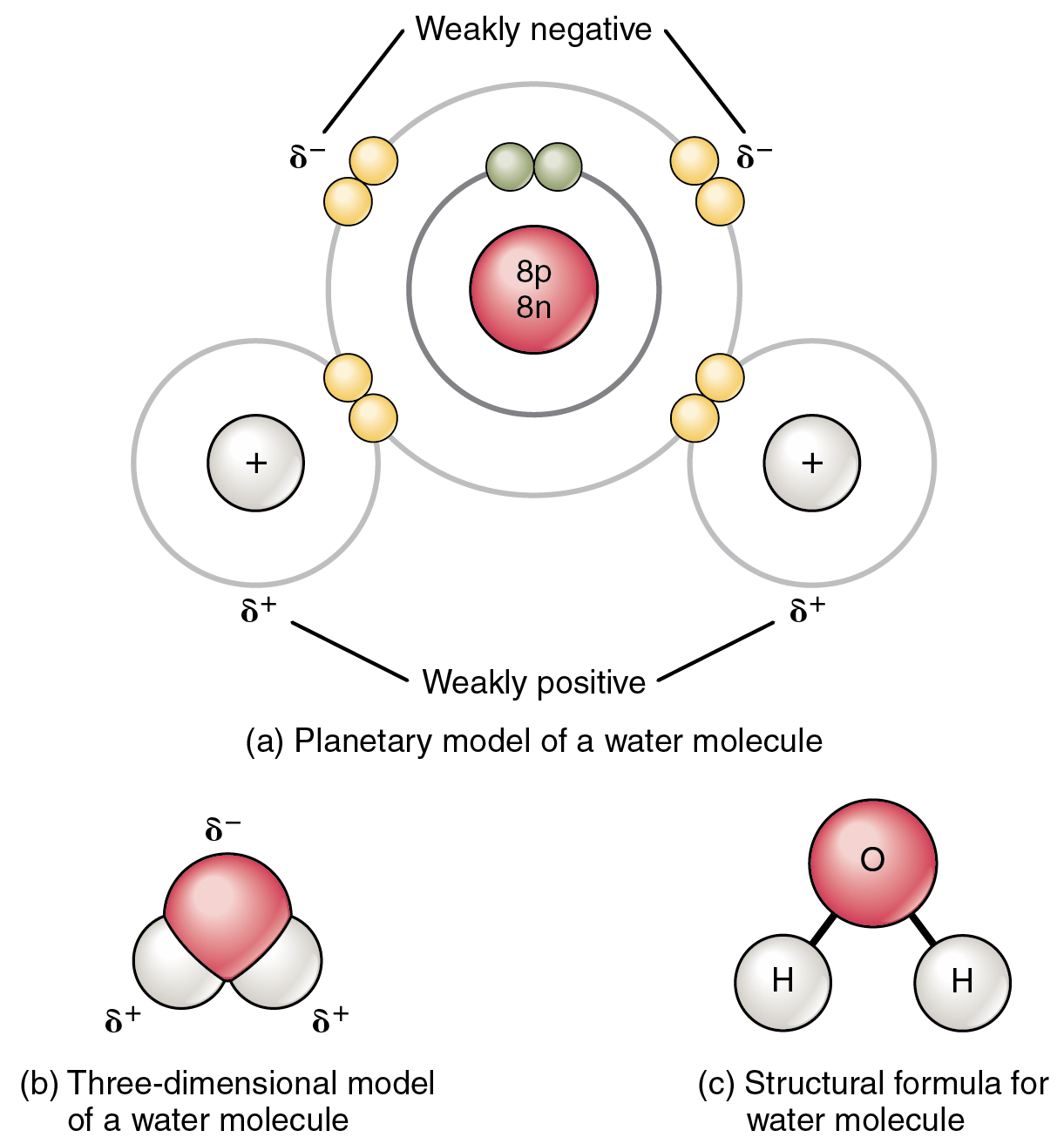
This figure shows the structure of a water molecule. The top panel
Polar Bond
How does a polar bond differ from a covalent bond
Related Post:
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)