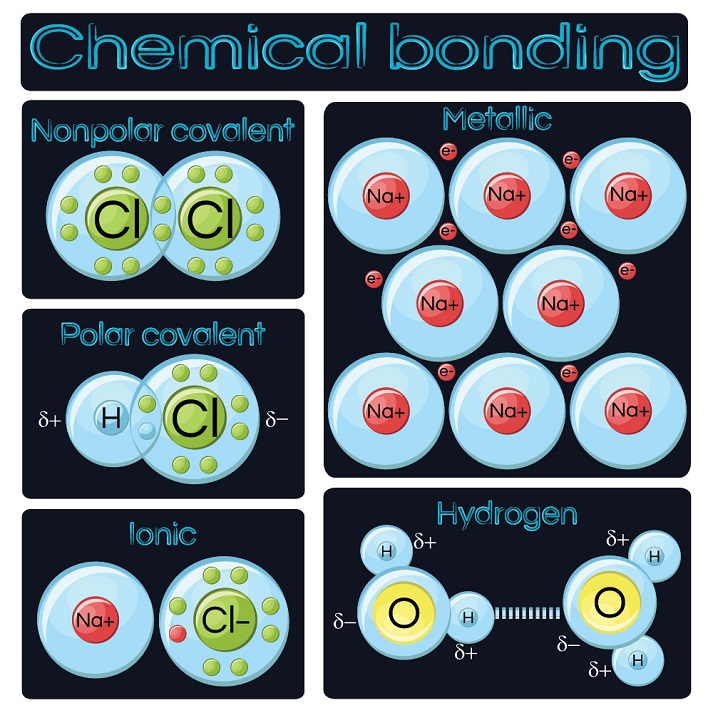
What Type Of Elements Form Ionic Bonds
What Type Of Elements Form Ionic Bonds - 1) for example, consider na and cl. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Ionic compounds generally form from metals and nonmetals. Web ions and ionic bonds some atoms become more stable by gaining or losing an entire electron (or several electrons). In general, covalent bonds form between. Elements with differences of electronegativity of 2.0 or more. When they do so, atoms form ions, or charged particles. Such a bond forms when the valence (outermost) electrons of one atom are transferred. Web which elements form ionic bonds? Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web which elements form ionic bonds? 3) last example, mg and cl. Elements with differences of electronegativity of 2.0 or more. Ionic bonds result from the attraction between oppositely charged ions. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). The resulting compound is called an ionic. In general, covalent bonds form between. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Elements with differences of electronegativity of 2.0 or more. When they do so, atoms form. It is a type of chemical bond that. Such a bond forms when the valence (outermost) electrons of one atom are transferred. Web the formation of ionic compounds. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic.. It is a type of chemical bond that. When they do so, atoms form ions, or charged particles. A metal (which forms the cations) and a nonmetal (which forms the anions). A metal (which forms the cations) and a nonmetal (which forms the anions). Elements with differences of electronegativity of 2.0 or more. The resulting compound is called an ionic. Ionic bonding is the complete transfer of valence electron (s) between atoms. Web predicting bond type (electronegativity) one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. In general, covalent bonds form between. Web we would like to show you a description. Web the formation of ionic compounds. Elements with differences of electronegativity of 2.0 or more. Ionic compounds generally form from metals and nonmetals. Web one way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. 2) another example, magnesium and oxygen. Ionic bonding is the complete transfer of valence electron (s) between atoms. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. 2) another example, magnesium and oxygen. Such a bond forms when the valence (outermost) electrons of. Binary ionic compounds are composed of just two elements: Web ionic and covalent bonds. Web the formation of ionic compounds. Web ions and ionic bonds some atoms become more stable by gaining or losing an entire electron (or several electrons). A metal (which forms the cations) and a nonmetal (which forms the anions). Binary ionic compounds are composed of just two elements: Covalent bonding involves the sharing of electrons between two or more atoms. Web compounds that contain ions are called ionic compounds. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web one type of chemical bond is an ionic bond. 2) another example, magnesium and oxygen. Web one type of chemical bond is an ionic bond. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). A metal (which forms the cations) and a nonmetal (which forms the anions). These ions are created by the transfer of valence. Ionic bonding is the complete transfer of valence electron (s) between atoms. These ions are created by the transfer of valence. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web the formation of ionic compounds. 2) another example, magnesium and oxygen. 1) for example, consider na and cl. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web we would like to show you a description here but the site won’t allow us. 3) last example, mg and cl. Ionic compounds generally form from metals and nonmetals. Web predicting bond type (electronegativity) one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. In general, covalent bonds form between. Covalent bonding involves the sharing of electrons between two or more atoms. When they do so, atoms form ions, or charged particles. Binary ionic compounds are composed of just two elements: Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Elements with differences of electronegativity of 2.0 or more. A metal (which forms the cations) and a nonmetal (which forms the anions). Web the formation of ionic compounds. Web compounds that contain ions are called ionic compounds.Ionic Bond Definition, Types, Properties & Examples
Ionic bonding Wikipedia
chemical bonding Definition, Types, & Examples Britannica
Ionic Bonding Presentation Chemistry
Ionic Bond Definition, Types, Properties & Examples
ionic bond Definition, Properties, Examples, & Facts Britannica
Chemical Bonds
Ionic Bond Definition, Types, Properties & Examples
Metallic Chemical Bonds Educational Resources K12 Learning, Chemistry
Ionic Bond Definition, Types, Properties & Examples
Related Post:



.PNG)


.PNG)