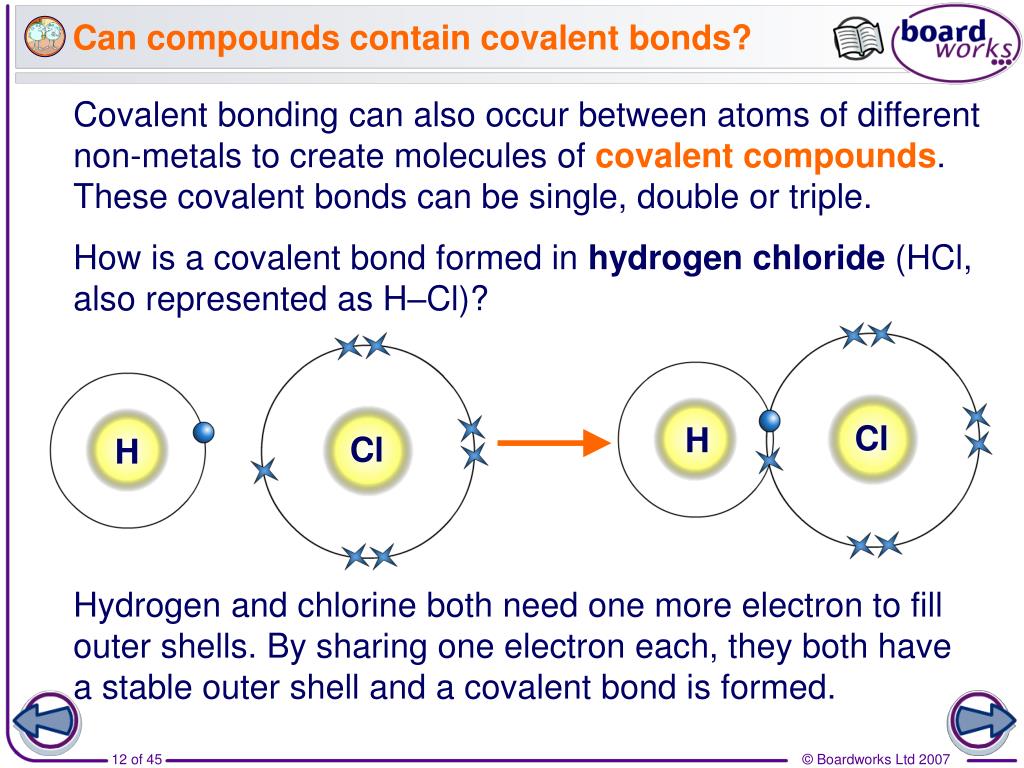
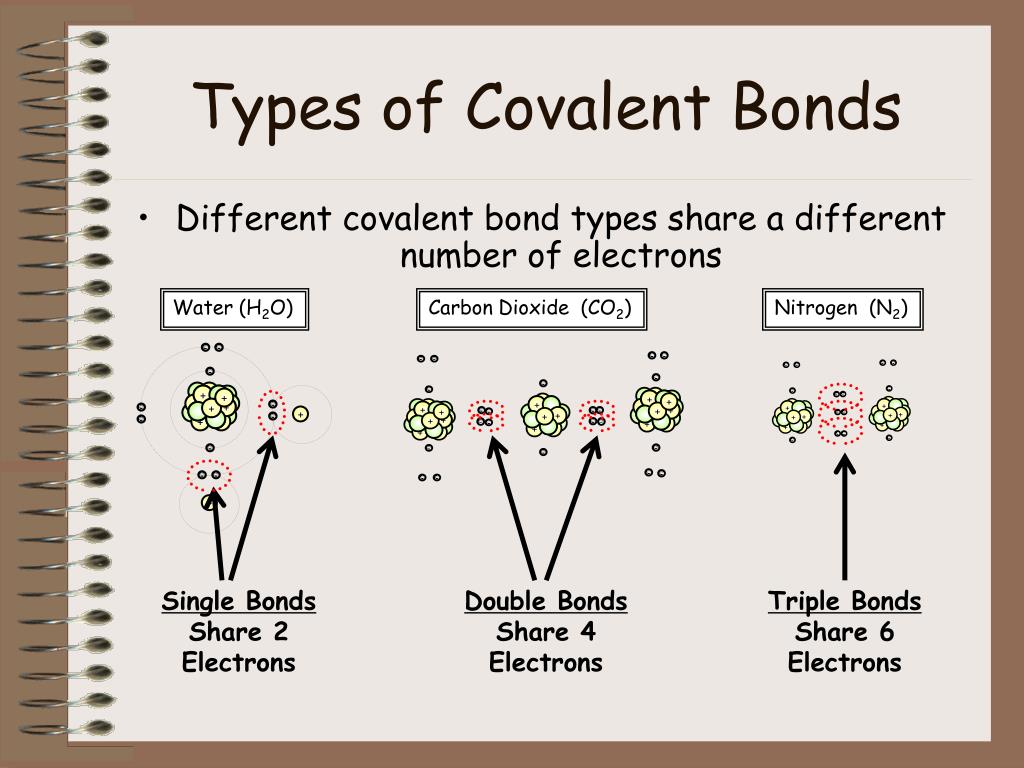
What Type Of Atoms Form Covalent Bonds
What Type Of Atoms Form Covalent Bonds - Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. In general, bonds are considered to be covalent if the electronegativity difference. Web covalent bond is formed when two atoms exchange one or more pairs of electrons. Web covalent bond definition. Is determined by the distance at which the lowest potential energy is achieved. A polar covalent bond is a covalent. Do a covalent bond should necessarily have a difference in their. In ionic bonding, atoms transfer electrons to each other. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. And group 7a form one bond. Web covalent bonds involve the sharing of electron pairs between atoms. Web typically, the atoms of group 4a form 4 covalent bonds; Web the two most basic types of bonds are characterized as either ionic or covalent. And group 7a form one bond. The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the. Web figure 3.3.1 3.3. Web typically, the atoms of group 4a form 4 covalent bonds; Web formation of covalent bonds. Ad study.com has been visited by 100k+ users in the past month Single and multiple covalent bonds. Group 6a form 2 bonds; Do a covalent bond should necessarily have a difference in their. Web the two most basic types of bonds are characterized as either ionic or covalent. When the participating atoms share the electrons equally, a covalent bond is formed. Covalent bonds form between atoms of nonmetallic elements. Web typically, the atoms of group 4a form 4 covalent bonds; Group 6a form 2 bonds; Single and multiple covalent bonds. Group 5a form 3 bonds; Ad explore a wide variety of books in science genre from authors around the globe. Web the two most basic types of bonds are characterized as either ionic or covalent. For example, the hydrogen molecule, h 2,. Single and multiple covalent bonds. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web apr 1, 2014. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two. For example, the hydrogen molecule, h 2,. Web covalent bonds and ionic bonds are types of atomic bonds. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. In this class, we will not discuss the option. The best guide to the covalent or ionic character of a. The electrons involved are in the outer shells of the atoms. These bonds are different in their properties and structure. The potential energy of two separate hydrogen atoms (right) decreases as. Web typically, the atoms of group 4a form 4 covalent bonds; The electrons involved are in the outer shells of the atoms. Covalent bonds include pairs of electrons by two atoms. In this class, we will not discuss the option. Web covalent bonds involve the sharing of electron pairs between atoms. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. Web apr 1, 2014. Web covalent bond definition. The electrons involved are in the outer shells of the atoms. For example, the hydrogen molecule, h 2,. An atom that shares one or more of its. Web covalent bond is formed when two atoms exchange one or more pairs of electrons. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. These bonds are different in their properties and structure. Predicting bond type (metals vs. A polar covalent bond is a covalent. The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the single electrons on each atom. In ionic bonding, atoms transfer electrons to each other. Ad study.com has been visited by 100k+ users in the past month Web two different atoms can also share electrons and form covalent bonds. Do a covalent bond should necessarily have a difference in their. Web the two most basic types of bonds are characterized as either ionic or covalent. Group 6a form 2 bonds; Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. The electrons involved are in the outer shells of the atoms. An atom that shares one or more of its. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. And group 7a form one bond. Predicting bond type (metals vs. Web covalent bond is formed when two atoms exchange one or more pairs of electrons. Covalent bonds form between atoms of nonmetallic elements. Understand the complexities of scientific topics with amazing books. The name of such paired electrons is bonding pair. Web covalent bonds involve the sharing of electron pairs between atoms. Group 5a form 3 bonds; Single and multiple covalent bonds.Covalent Bond Biology Dictionary
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Covalent Bonding (Biology) — Definition & Role Expii
The top panel in this figure shows two hydrogen atoms sharing two
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download
covalent bond Definition, Properties, Examples, & Facts Britannica
chemical bonding Definition, Types, & Examples Britannica
Covalent Bond Definition, Types, and Examples
Related Post: