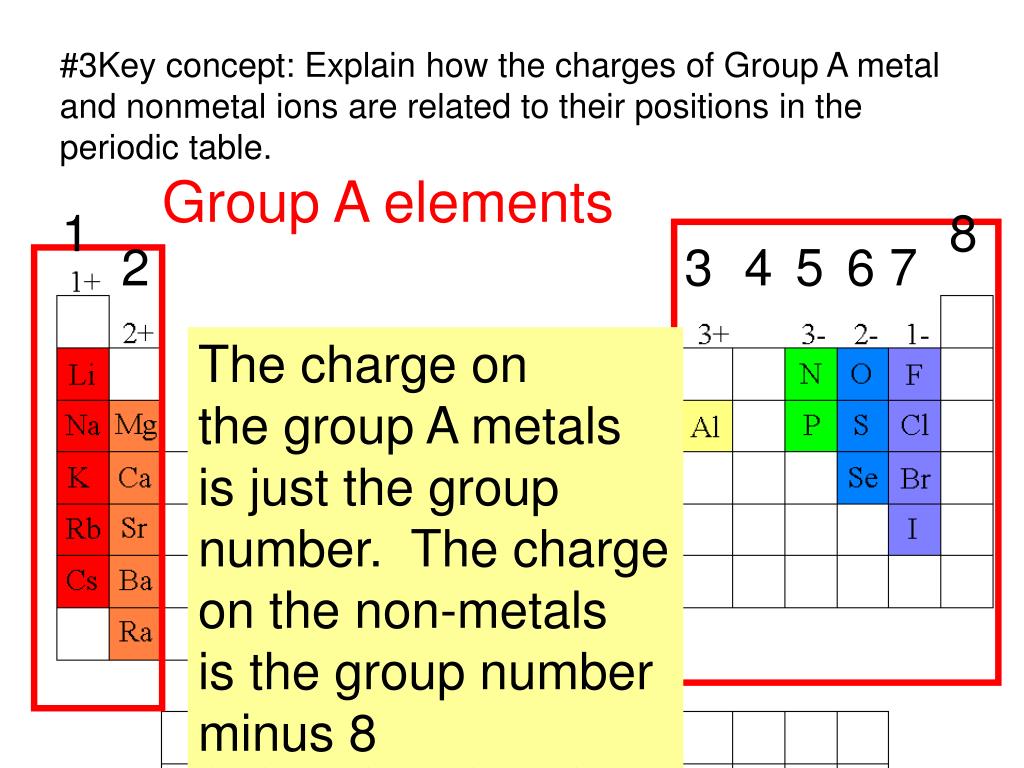
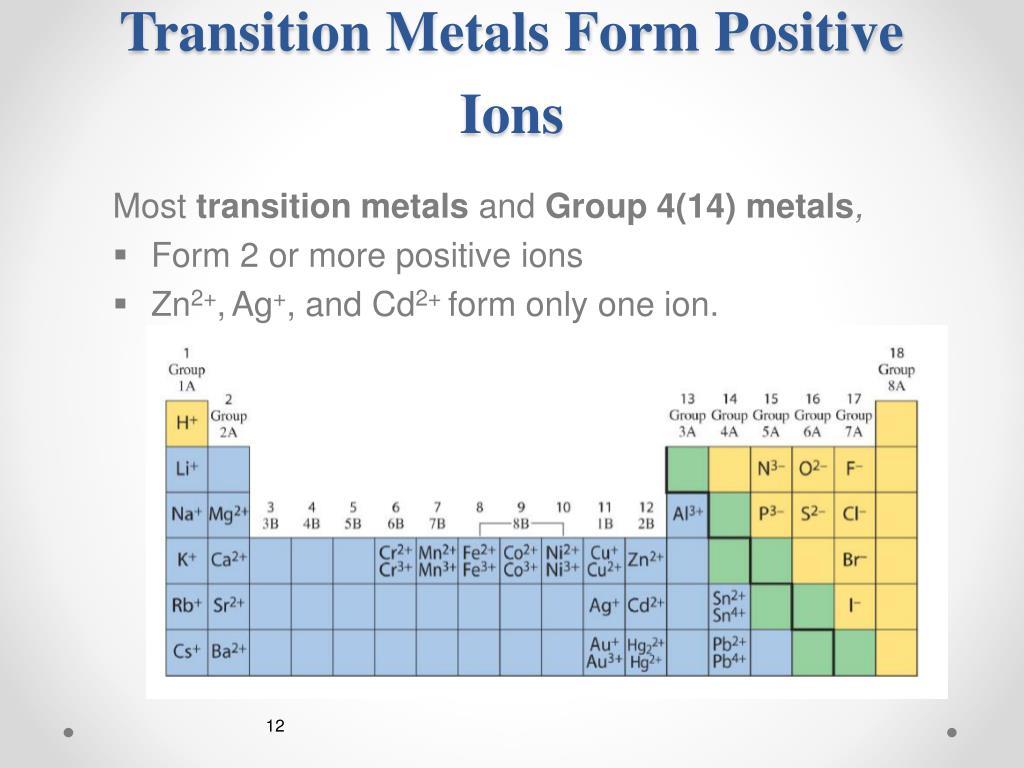
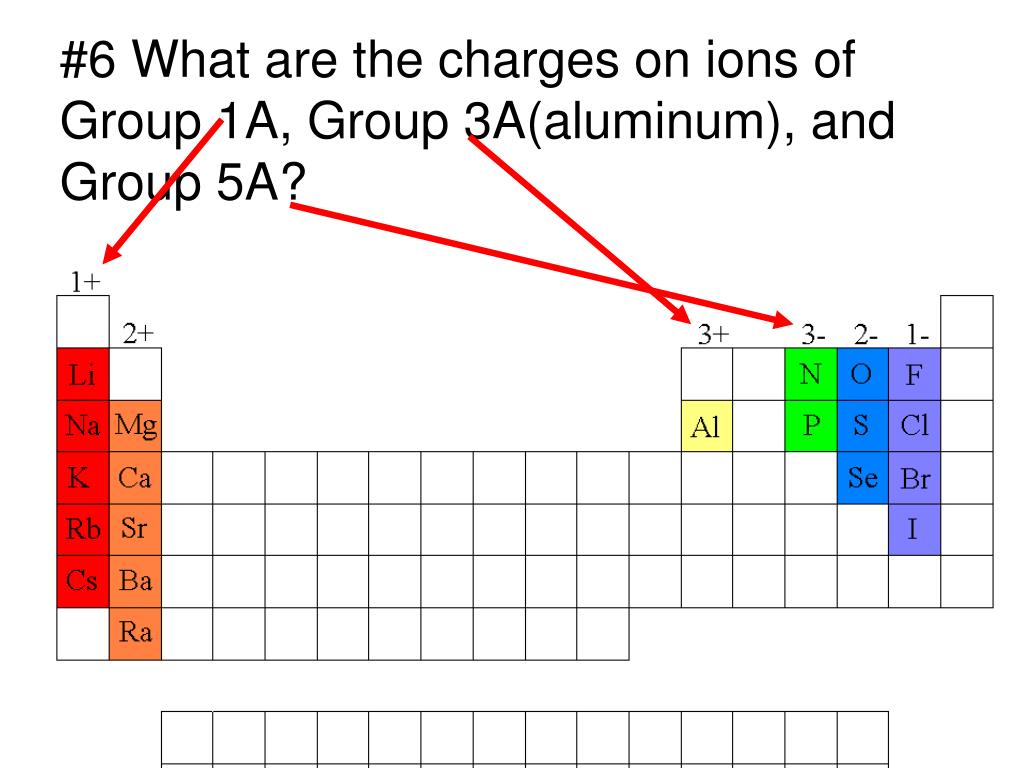
What Kind Of Ions Do Metals Form
What Kind Of Ions Do Metals Form - What type of ions do transition metals form? Positively charged ions are called cations; Iron can form fe 2+ and. Web metals form positive ions (cations). “an ion is a small electrically charged particle. What type of ions do transition metals form? Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web transitional metal ions, such as cu 2+, zn 2+, ni 2+, and co 2+, can form coordination bonds with the imidazolyl group of histidine. Option (d) is the correct answer. Web the most common type of ionic bonding is seen in compounds of metals and nonmetals (except noble gases, which rarely form chemical compounds). Option (d) is the correct answer. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. Ions are single charged atoms (simple ions) or small. What type of ions do transition metals form? Option (d) is the correct answer. The ions formed have full. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Web metal atoms lose electrons from their outer shell when they form ions: Metals are the species which contain more number of electrons. Web metals form positive ions (cations). Web metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web metals form positive ions (cations). Positively charged ions are called cations; The scientific name for positively charged ions is cations. So how do you know what kind of bond an atom will make? What type of ions do transition metals form? Web ionic bonds form when two or more ions come together and are held together by charge differences. Web metal atoms lose electrons from their outer shell when they form ions: A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. So how do you know what kind of. Ions are single charged atoms (simple ions) or small charged “molecules” (polyatomic ions).” simple. When a specie loses electrons then it acquires. The scientific name for positively charged ions is cations. React to form 1+ ions (e.g. Web metals form positive ions (cations). Option (d) is the correct answer. Web metals form positive ions (cations). Metals are the species which contain more number of electrons. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web metal atoms lose electrons from their outer shell when they form ions: Once a protein and a solid surface form. Positively charged ions are called cations; Web metal atoms lose electrons to form positive ions ( cations. React to form ions with different charges e.g. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. The ions formed have full. React to form ions with different charges e.g. What type of ions do transition metals. In these compounds you have the cation, often an alkali metal itself, stabilized in. Option (d) is the correct answer. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web ionic bonds form when two or more ions come together and are held together by charge differences. Option (d) is the correct answer. What type of ions do transition metals form? Web the most common type of. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web metal atoms lose electrons from their outer shell when they form ions: The scientific name for positively charged ions is cations. The type of ions that metals form are called positively charged ions. Positively charged ions are called cations; Iron can form fe 2+ and. Web metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Metals are the species which contain more number of electrons. The ions formed have full. Web the most common type of ionic bonding is seen in compounds of metals and nonmetals (except noble gases, which rarely form chemical compounds). In these compounds you have the cation, often an alkali metal itself, stabilized in. Option (d) is the correct answer. So how do you know what kind of bond an atom will make? Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. The ions are positive, because they have more. When a specie loses electrons then it acquires. What type of ions do transition metals form? React to form 1+ ions (e.g.Examples of Ionic Compoiunds YouTube
PPT 1 Name the ions formed by these elements and classify them as
Chem matters ch6_ionic_bond
Ions
PPT Naming Ionic Compounds PowerPoint Presentation, free download
PPT 1 Name the ions formed by these elements and classify them as
PPT 1 Name the ions formed by these elements and classify them as
Chem matters ch6_ionic_bond
Chem Ions Scientific Tutor
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free
Related Post: