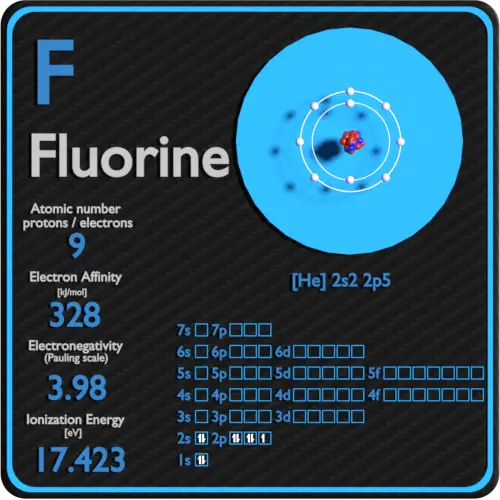
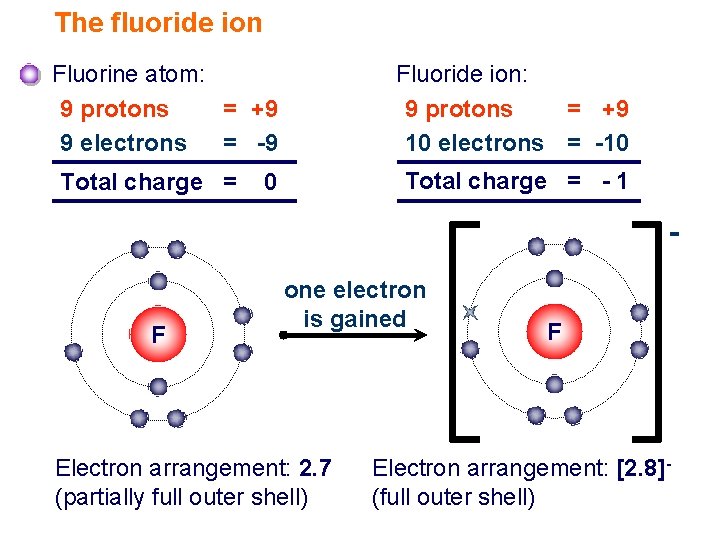
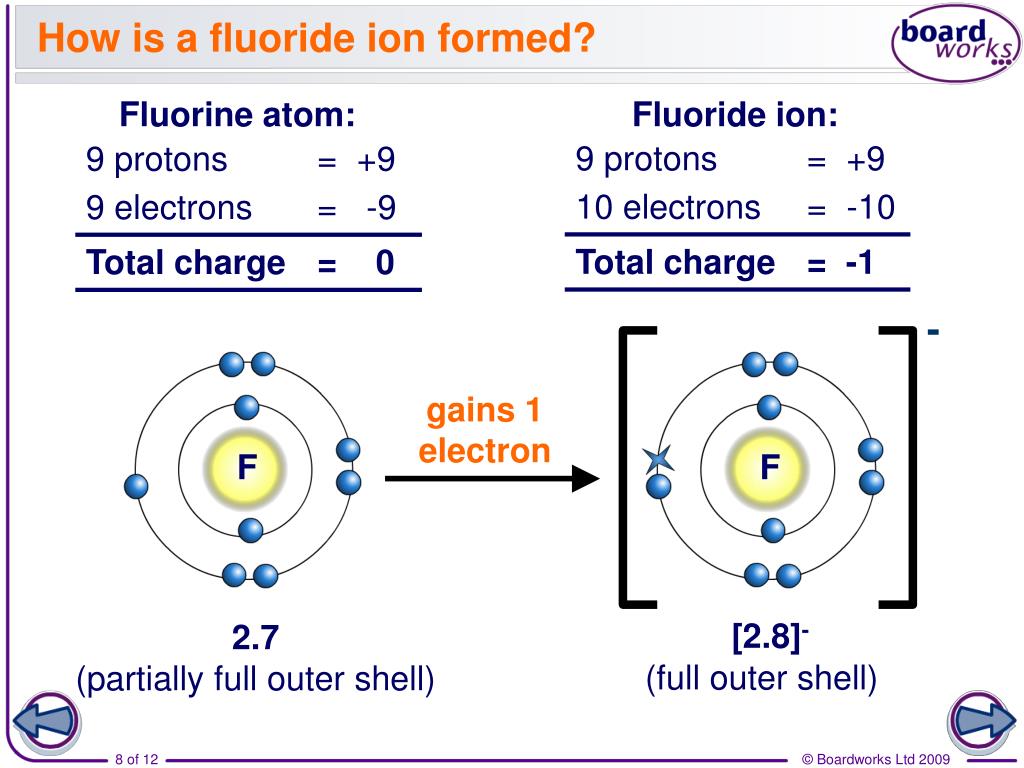
What Is The Most Common Ionic Form Of Fluorine
What Is The Most Common Ionic Form Of Fluorine - Web the aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. That means, 1680.6 kj energy is required to extract an electron from one mole of. However, the radioactive isotopes 17 f, 18 f, and 20 f are known. What is the most common ionic form of fluorine? It differs from this value in elemental fluorine, where the atoms are bonded to each other and thus at oxidation state 0, and a few polyatomic ions: Learn more about the properties of halogens in this article. This combination is written as \(\ce{alf3}\). Fluorine has been known to form compounds with rare gases, including xenon, radon, and krypton. Predict the products and write the correct balanced equation for the single replacement reaction. Synthetic routes to fluorocarbon compounds; What is the most common ionic form of fluorine? It is possible that fluorine can substitute for hydrogen in organic reactions. Web the most common fluorine minerals are fluorite, fluorspar and cryolite, but it is also rather widely distributed in other minerals. Since fluorine forms an anion, metal cations can bond with it to form ionic compounds. When forming ions,. We can use this method to predict the charges of ions in ionic compounds. Since fluorine forms an anion, metal cations can bond with it to form ionic compounds. These have the cubic arrangement of sodium chloride and analogous chlorides. It is the lightest halogen. Web fluorine (f 2), composed of two fluorine atoms, combines with all other elements except. Fluorine is made by the electrolysis of a solution of potassium hydrogendifluoride (khf2) in anhydrous hydrofluoric acid. Web figure 3.7 depicts the most common ionic states of the elements and shows the two most common ionic states for elements that can form more than one ion. The compound fbr is not an ionic compound and is not likely to form. Free fluorine has a characteristic. Web using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for fluorine (f). Fluoride salts typically have distinctive bitter tastes, and are odorless. It is possible that fluorine can substitute for hydrogen in organic reactions. Web fluorine (f 2), composed of. It is the lightest halogen. Web figure 3.7 depicts the most common ionic states of the elements and shows the two most common ionic states for elements that can form more than one ion. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Elemental fluorine (f 2) is the most. Web the most common fluorine minerals are fluorite, fluorspar and cryolite, but it is also rather widely distributed in other minerals. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The oxygen atom has a 2− charge as an ion. Web fluorine (f 2), composed of two fluorine atoms, combines. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Web using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for fluorine (f). An ionic bond is defined as an electrostatic attraction between a metal cation and. Predict the products and write the correct balanced equation for the single replacement reaction. Fluorine is the most reactive and the most electronegative of all the elements. ), whose salts are typically white or colorless. Web the aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Since fluorine forms an anion, metal. The oxygen atom has a 2− charge as an ion. An ionic bond is defined as an electrostatic attraction between a metal cation and a nonmetal anion. Fluorine is the most reactive and the most electronegative of all the elements. ), whose salts are typically white or colorless. Figure 3.7 common ionic states of the elements. Web the aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. What is wrong with the student's prediction of the products in the above reaction? There is only one stable, naturally occurring isotope of fluorine: Fluorine is made by the electrolysis of a solution of potassium hydrogendifluoride (khf2) in anhydrous hydrofluoric acid.. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Metals will form ionic bonds with fluorine. That means, 1680.6 kj energy is required to extract an electron from one mole of. Web alkali metals form ionic and highly soluble monofluorides; The oxygen atom has a 2− charge as an ion. Some metals, such as nickel, are quickly covered by a fluoride layer, which prevents further attack of. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. What is the most common ionic form of fluorine? Web some common ionic reactions of fluorine are: Web fluorine is the most electronegative element. Fluorine is the most reactive and the most electronegative of all the elements. This combination is written as \(\ce{alf3}\). Web the halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i), astatine (at), and tennessine (ts). An ionic bond is defined as an electrostatic attraction between a metal cation and a nonmetal anion. For elements that have more than one common ionic state, both states are listed. It is the 13th most common element in the earth’s crust. Fluoride salts typically have distinctive bitter tastes, and are odorless. The compound fbr is not an ionic compound and is not likely to form in a single replacement reaction. Fluorine is a chemical element that in pure form occurs as a dimer of two fluorine atoms, f 2. Web web fluoride is the ionic form of the element fluorine, and it inhibits or reverses the initiation and progression of dental caries (tooth decay) and stimulates new bone formation.PPT FLUORINE PowerPoint Presentation, free download ID2034879
How To Find Electron Configuration For Fluorine Dynamic Periodic
Covalent Bonding (Molecules) Presentation Chemistry
Fluorine F (Element 9) of Periodic Table Elements Flash Cards
Fluorine Periodic Table and Atomic Properties
Ionic Bonding Elements are the simplest substances There
Fluorine Facts, Symbol, Discovery, Properties, Uses
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Fluorine, atomic structure Stock Image C018/3690 Science Photo Library
Fluorine Uses, Properties, & Facts Britannica
Related Post:

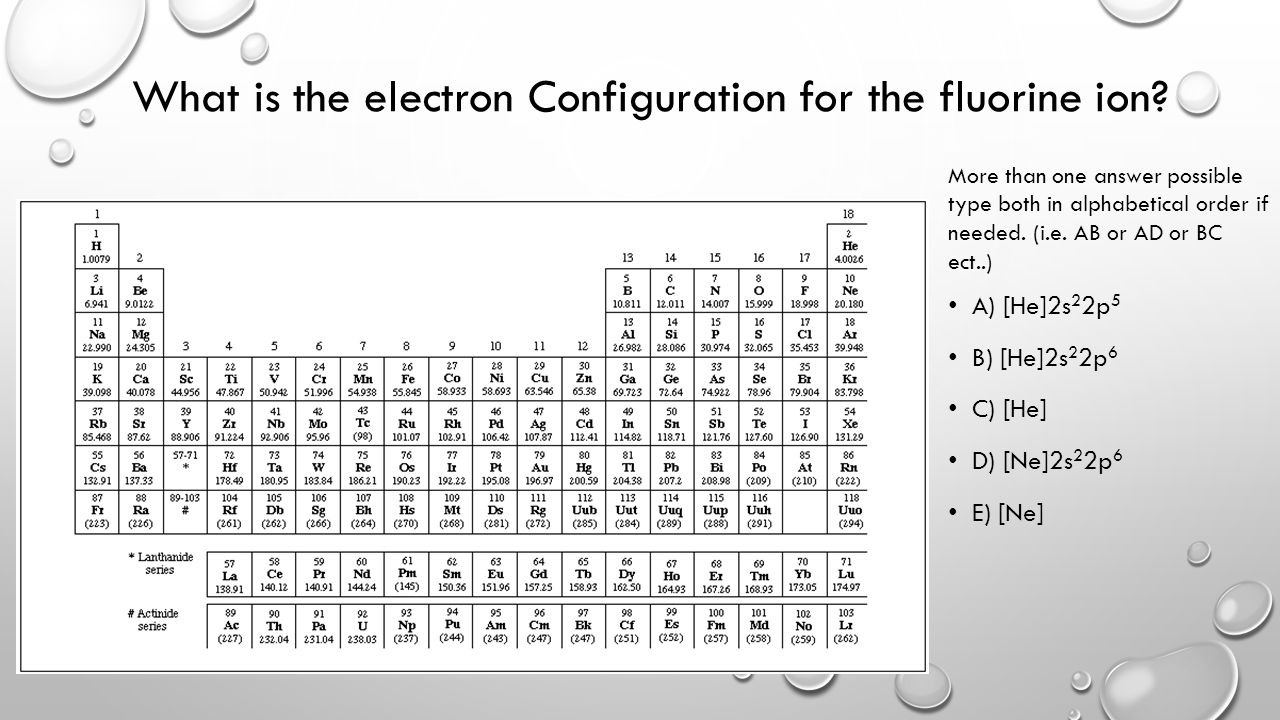
.PNG)