What Is Necessary For A Metallic Bond To Form
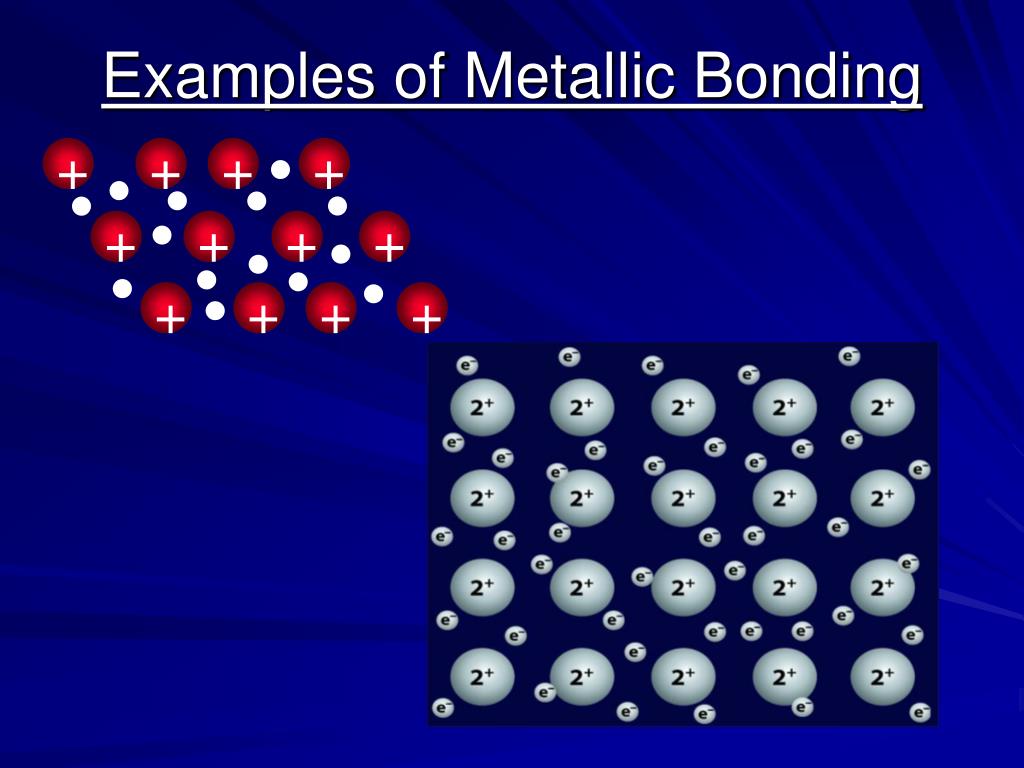
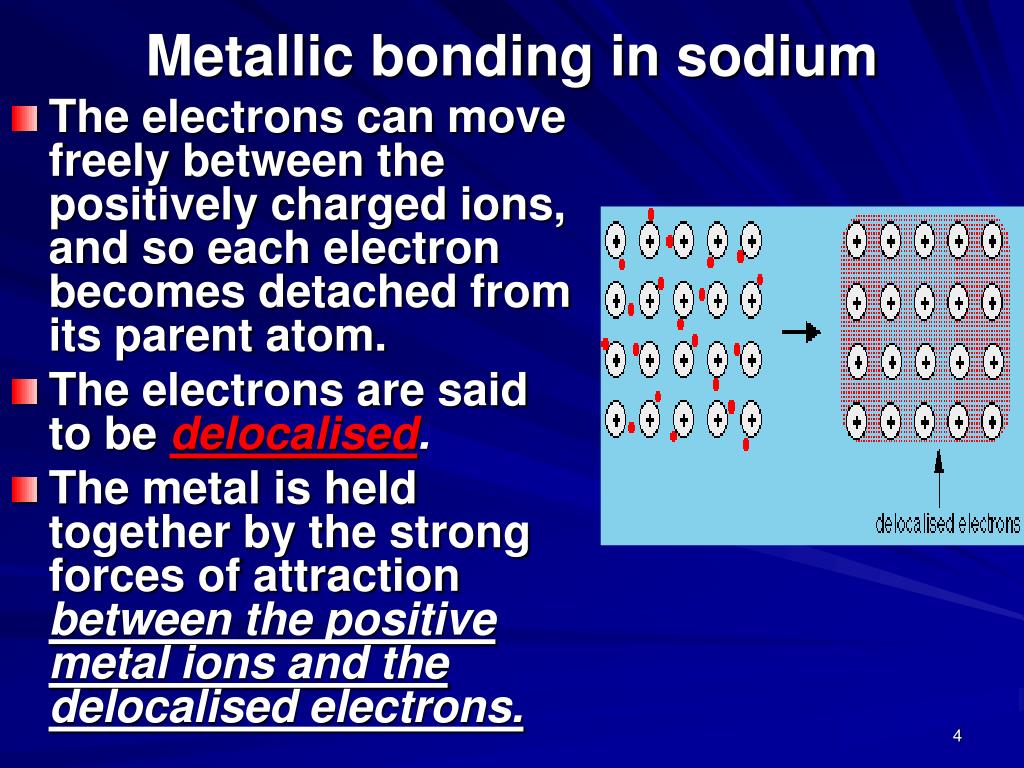
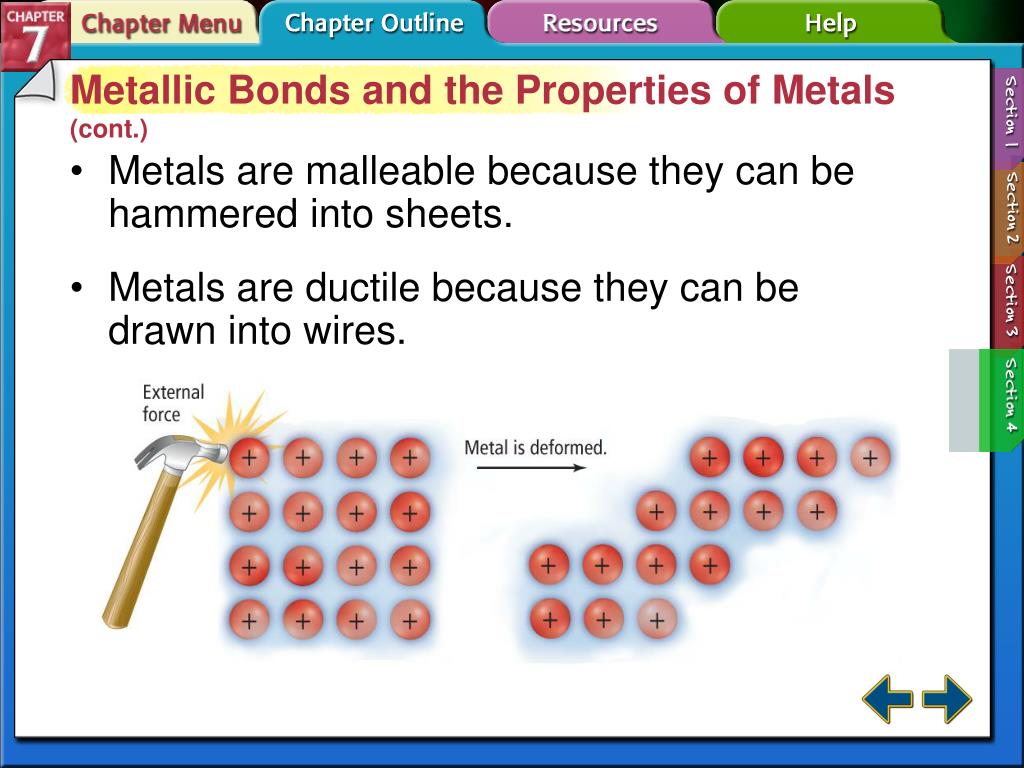
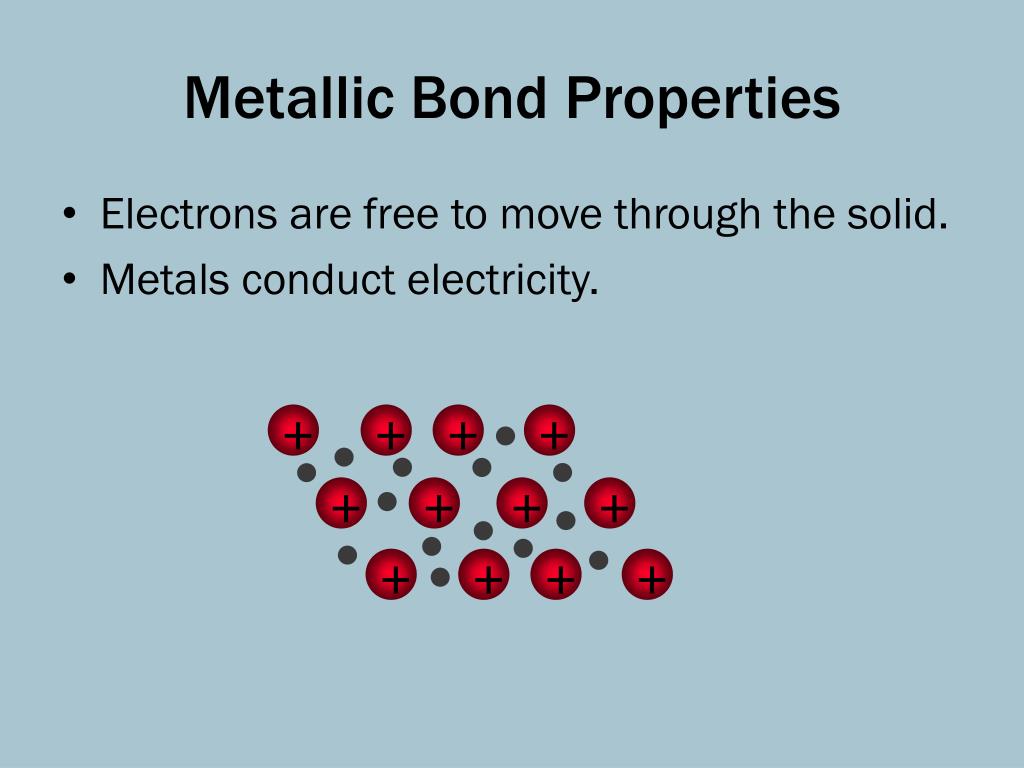
What Is Necessary For A Metallic Bond To Form - Web we would like to show you a description here but the site won’t allow us. What is necessary for a metallic bond to form? A more complex model is needed to explain the bonding in. The nature of metallic bonding accounts for many of the physical properties of metals, such as conductivity and malleability. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. Web metallic bonds are formed when the charge is spread over a larger distance as compared to the size of single atoms in solids. A metallic bonding theory must. Properties attributed by metallic bonding. Metallic bonding is a type of chemical bonding between two or more metal atoms, which arises from the attraction between positively charged metal nuclei. Web metallic bonds are strong and require a great deal of energy to break, and therefore metals have high melting and boiling points. In a sample of metal, the valence electrons detach from the atoms and are. A more complex model is needed to explain the bonding in. Web because they show no tendency to form negative ions, the kind of bonding present in ionic solids can immediately be ruled out. Web ordinary covalent bonding can also be ruled out. Web metallic bonding. Web metallic bonds are strong and require a great deal of energy to break, and therefore metals have high melting and boiling points. Both metallic and covalent bonding. For a metallic bond to form the atoms of the metal element must be able to form a sea of delocalized valence electrons that through their. These free electrons are called delocalized. This model represents metal crystals as being made up of. The metallic elements have empty. Web metallic bonds are formed when the charge is spread over a larger distance as compared to the size of single atoms in solids. Properties attributed by metallic bonding. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force. What is required for a metallic bond to form? Web metallic bonds are strong and require a great deal of energy to break, and therefore metals have high melting and boiling points. Mostly, in the periodic table, left. Both metallic and covalent bonding. In a sample of metal, the valence electrons detach from the atoms and are. Web the metallic bond is a unique type of chemical bond found in metal elements. What is required for a metallic bond to form? For a metallic bond to form the atoms of the metal element must be able to form a sea of delocalized valence electrons that through their. Mostly, in the periodic table, left. Web metallic bonding is. Metallic bonds occur among metal atoms. Web the metallic bond is a unique type of chemical bond found in metal elements. What is a metallic bond? Web metallic bonds are formed when the charge is spread over a larger distance as compared to the size of single atoms in solids. In a sample of metal, the valence electrons detach from. In a sample of metal, the valence electrons detach from the atoms and are. What is a metallic bond? Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. The metallic elements have empty. Mostly, in the periodic table, left. What is a metallic bond? What is necessary for a metallic bond to form? This model represents metal crystals as being made up of. Web the metallic bond is a unique type of chemical bond found in metal elements. A metallic bonding theory must explain how so. Web we would like to show you a description here but the site won’t allow us. Each covalent bond would require one electron from each atom, and no metal has 12 valence electrons. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of. The metallic elements have empty. A metallic bonding theory must. What is required for a metallic bond to form? Metallic bonds occur among metal atoms. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. Properties attributed by metallic bonding. Web metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Mostly, in the periodic table, left. A metallic bonding theory must explain how so. These free electrons are called delocalized because they are not. Web metallic bonds are formed when the charge is spread over a larger distance as compared to the size of single atoms in solids. Each covalent bond would require one electron from each atom, and no metal has 12 valence electrons. Web the metallic bond is a unique type of chemical bond found in metal elements. In a sample of metal, the valence electrons detach from the atoms and are. A metallic bonding theory must. Metallic bonds result from the electrostatic attraction between metal cations and delocalized electrons. Web because they show no tendency to form negative ions, the kind of bonding present in ionic solids can immediately be ruled out. The atoms should be in close proximity. A more complex model is needed to explain the bonding in. Web metallic bonds are strong and require a great deal of energy to break, and therefore metals have high melting and boiling points. This model represents metal crystals as being made up of. The atoms should be far apart. Web ordinary covalent bonding can also be ruled out. All elements tend to try and get eight electrons in their outermost shell, or. What is required for a metallic bond to form?PPT Unit 4 Metallic Bonding PowerPoint Presentation, free download
Metallic Bonding Definition, Types, & Properties (2022)
Metallic bond Properties, Examples, & Explanation Britannica
Metallic Bond — Formation & Compounds Expii
Metallic Bonding Definition, Types, & Properties (2022)
PPT METALLIC BOND PowerPoint Presentation, free download ID4554784
What is a metallic bond and how does it form Metallic Bonding
PPT Metallic Bonding and Naming of Ionic Bonds PowerPoint
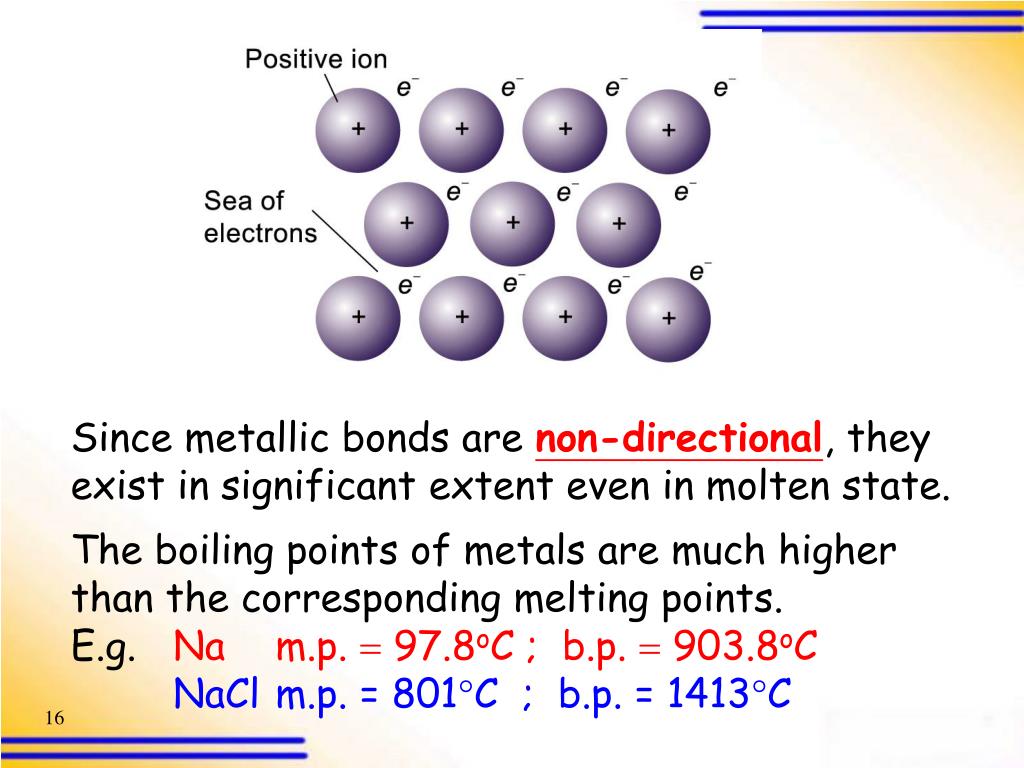
PPT Metallic Bonding PowerPoint Presentation, free download ID2696346
PPT Metallic Bonding PowerPoint Presentation, free download ID711524
Related Post: