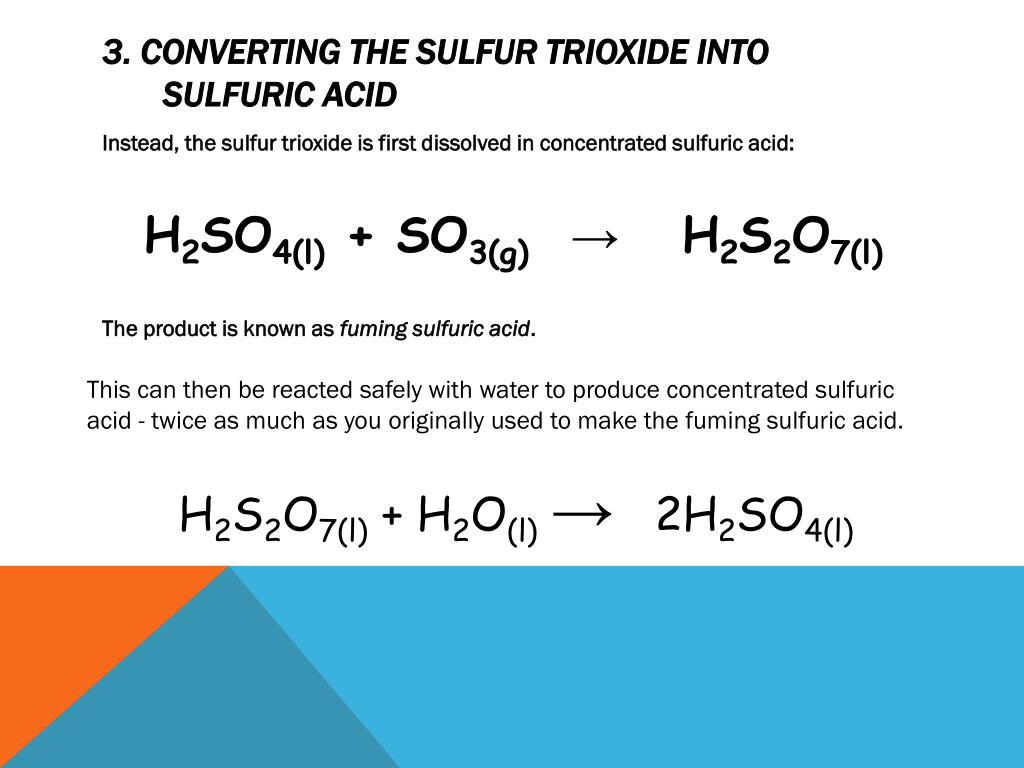
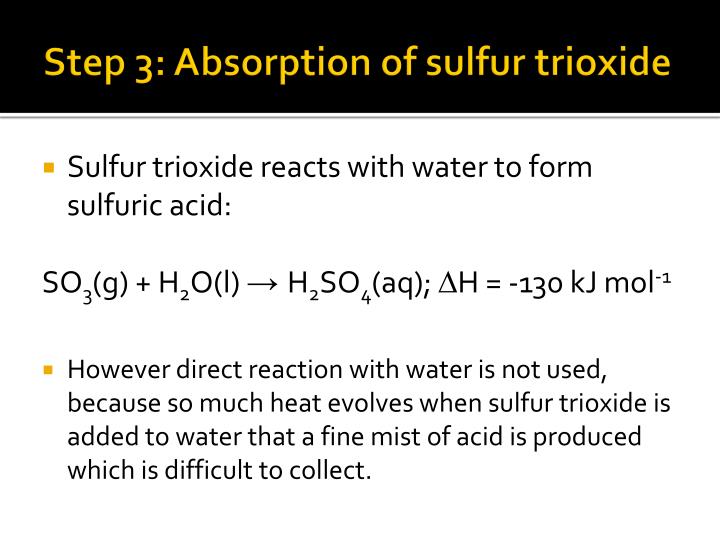
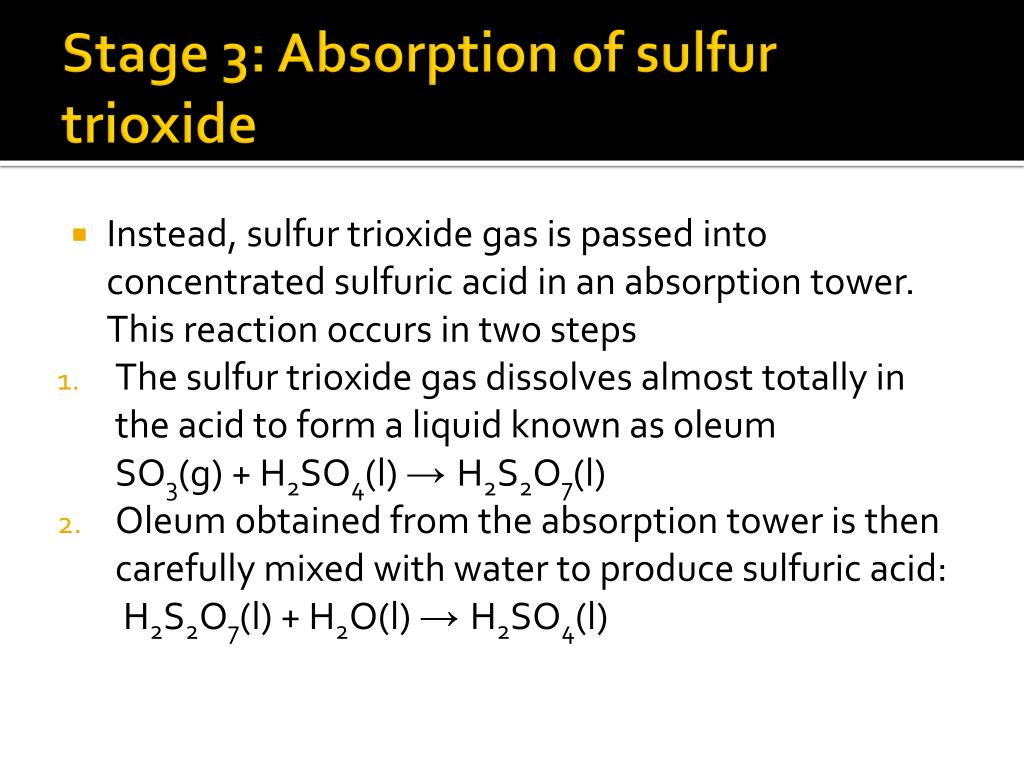
Sulfur Trioxide Reacts With Water To Form
Sulfur Trioxide Reacts With Water To Form - Web in contact process.hot catalyst, unite to form sulfur trioxide, which in turn combines with water to make sulfuric acid. It reacts violently with water to produce highly corrosive sulfuric acid. One origin of so3 is the combustion of sulfur, which is present in small quantities of coal,. Web in the air, sulfur trioxide can be formed slowly from sulfur dioxide. Web sulfur trioxide (so3) dissolves in and reacts with water to form an aqueous solution of sulfuric acid (h2so4). Sulfur trioxide reacts violently with water to produce a fog of concentrated sulfuric acid droplets. \[ \underbrace{\ce{2 so2(g) + o2(g). The vapor in equilibrium with the solution contains both so_3. Web sulfur trioxide reacts with water to form sulfuric acid, a major contributor to acid rain. This is an unfortunately common reaction that occurs in the atmosphere in some places where. Both sulfur dioxide and sulfur trioxide react to form sulfuric. Web the reaction of sulfur trioxide with atmospheric water droplets results in sulfuric acid, a contributor to acid rain.according to the equation below, how many moles. Sulfur trioxide reacts violently with water to produce a fog of concentrated sulfuric acid droplets. 2so 2 (g) + o 2 (g) ⇌ 2so. Web sulfur dioxide and oxygen, \(\ce{so_2} + \ce{o_2}\), are reactants and sulfur trioxide, \(\ce{so_3}\), is the product. Web we would like to show you a description here but the site won’t allow us. Web sulfur trioxide is often a colorless or white solid that creates white fumes in the air and has strong reactions with water. Web sulfur trioxide (so_3). So3 + h2o → h2so4. Web sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain. The so 3 ···h 2 o + h 2 o reaction and the so 3 + (h 2 o) 2 reaction. Calculate the mass in grams of 3.65 × 1020 molecules of sulfur trioxide. One origin of. Calculate the mass in grams of 3.65 times 10^20 molecules of sulfur troxide. Web sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain. Both sulfur dioxide and sulfur trioxide react to form sulfuric. Web sulfur trioxide (so_3) dissolves in and reacts with water to form an aqueous solution of sulfuric acid (h_2so_4).. It reacts violently with water to produce highly corrosive sulfuric acid. One origin of so3 is the combustion of sulfur, which is present in small quantities of coal,. Web sulfur trioxide gas reacts with water to form sulfuric acid. Web in contact process.hot catalyst, unite to form sulfur trioxide, which in turn combines with water to make sulfuric acid. Web. Sulfur dioxide + oxygen ⇌ sulfur trioxide. Web in the second stage, sulfur dioxide reacts with more oxygen to make sulfur trioxide: Calculate the mass in grams of 3.65 × 1020 molecules of sulfur trioxide. The vapor in equilibrium with the solution contains. What is the balanced equation? Along with being an oxidizing agent, sulfur trioxide is highly corrosive. Sulfur trioxide reacts violently with water to produce a fog of concentrated sulfuric acid droplets. Web sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain. Web when sulfur trioxide gas reacts with water, a solution of sulfuric acid forms. Once formed,. 2so 2 (g) + o 2 (g) ⇌ 2so 3 (g) this reaction is. Sulfur dioxide + oxygen ⇌ sulfur trioxide. Web sulfur dioxide and oxygen, \(\ce{so_2} + \ce{o_2}\), are reactants and sulfur trioxide, \(\ce{so_3}\), is the product. This is an unfortunately common reaction that occurs in the atmosphere in some places where. Calculate the mass in grams of 3.65. Once formed, sulfur trioxide will react with water in the air to form sulfuric acid. Web sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain. One origin of so3 is the combustion of sulfur, which is present in small quantities of coal,. Web in the air, sulfur trioxide can be formed slowly. Web the reaction of sulfur trioxide with atmospheric water droplets results in sulfuric acid, a contributor to acid rain.according to the equation below, how many moles. Web we consider two mechanisms: What is the balanced equation? Once formed, sulfur trioxide will react with water in the air to form sulfuric acid. Calculate the mass in grams of 3.65 times 10^20. Both sulfur dioxide and sulfur trioxide react to form sulfuric. Web in contact process.hot catalyst, unite to form sulfur trioxide, which in turn combines with water to make sulfuric acid. Web in the air, sulfur trioxide can be formed slowly from sulfur dioxide. \[ \underbrace{\ce{2 so2(g) + o2(g). Web we consider two mechanisms: One origin of so3 is the combustion of sulfur, which is present in small quantities of coal,. Web sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain. The vapor in equilibrium with the solution contains both so_3. Given the atomic mass of hydrogen is 1 amu, the atomic mass of. Web when sulfur trioxide is formed, it can further react with the water particles present in the air to form sulfuric acid, which is the main component of acid rain. Web sulfur trioxide gas reacts with water to form sulfuric acid. Web sulfur trioxide reacts with water to form sulfuric acid, a major contributor to acid rain. What is the balanced equation? Web sulfur trioxide is often a colorless or white solid that creates white fumes in the air and has strong reactions with water. The so 3 ···h 2 o + h 2 o reaction and the so 3 + (h 2 o) 2 reaction. Along with being an oxidizing agent, sulfur trioxide is highly corrosive. Once formed, sulfur trioxide will react with water in the air to form sulfuric acid. Web sulfur trioxide (so_3) dissolves in and reacts with water to form an aqueous solution of sulfuric acid (h_2so_4). This is an unfortunately common reaction that occurs in the atmosphere in some places where. So3 + h2o → h2so4.PPT The Contact process PowerPoint Presentation, free download ID
Sulfur Trioxide And Water Make Sulfuric Acid YouTube
PPT Production of Sulfuric Acid PowerPoint Presentation ID2170530
PPT Production of Sulfuric Acid PowerPoint Presentation, free
Mechanism of Reaction of Sulfur Trioxide with Water to make Sulfuric
PPT LECTURE 9 SULFUR AND SULFURIC ACID PowerPoint Presentation, free
sulphur trioxide reacts with water to give sulphuric acid balance the
Mechanism of Reaction of Sulfur Trioxide with Water to make Sulfuric
Mechanism of sulfur trioxide reaction with water to make sulfuric acid
H2so4 Enthalpy Of Formation
Related Post: